Preparation of Chitin Nanofibers from Mushrooms
- PMID: 28824151
- PMCID: PMC5448680
- DOI: 10.3390/ma4081417
Preparation of Chitin Nanofibers from Mushrooms
Abstract
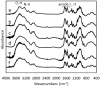
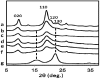
Chitin nanofibers were isolated from the cell walls of five different types of mushrooms by the removal of glucans, minerals, and proteins, followed by a simple grinding treatment under acidic conditions. The Chitin nanofibers thus obtained have a uniform structure and a long fiber length. The width of the nanofibers depended on the type of mushrooms and varied in the range 20 to 28 nm. The Chitin nanofibers were characterized by elemental analyses, FT-IR spectra, and X-ray diffraction profiles. The results showed that the α-chitin crystal structure was maintained and glucans remained on the nanofiber surface.
Keywords: chitin; mushrooms; nanofibers.
Figures


References
-
- Muzzarelli R.A.A. Chitin nanostructures in living organisms. In: Gupta S.N., editor. Chitin Formation and Diagnosis. Springer; New York, NY, USA: 2011.
-
- Kunzek H., Müller S., Vetter S., Godeck R. The significance of physico chemical properties of plant cell wall materials for the development of innovative food products. Eur. Food Res. Technol. 2002;214:361–376. doi: 10.1007/s00217-002-0487-0. - DOI
-
- Guillon F., Champ M. Structural and physical properties of dietary fibers, and consequences of processing on human physiology. Food Res. Int. 2000;33:233–245. doi: 10.1016/S0963-9969(00)00038-7. - DOI
-
- Ifuku S., Nogi M., Yoshioka M., Morimoto M., Yano H., Saimoto H. Fibrillation of dried chitin into 10–20 nm nanofibers by a simple method under acidic conditions. Carbohydr. Polym. 2010;81:134–139. doi: 10.1016/j.carbpol.2010.02.006. - DOI
LinkOut - more resources
Full Text Sources

