Body Temperature Measurements for Metabolic Phenotyping in Mice
- PMID: 28824441
- PMCID: PMC5534453
- DOI: 10.3389/fphys.2017.00520
Body Temperature Measurements for Metabolic Phenotyping in Mice
Abstract
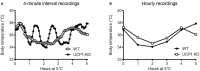
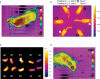
Key Points Rectal probing is subject to procedural bias. This method is suitable for first-line phenotyping, provided probe depth and measurement duration are standardized. It is also useful for detecting individuals with out-of-range body temperatures (during hypothermia, torpor).The colonic temperature attained by inserting the probe >2 cm deep is a measure of deep (core) body temperature.IR imaging of the skin is useful for detecting heat leaks and autonomous thermoregulatory alterations, but it does not measure body temperature.Temperature of the hairy or shaved skin covering the inter-scapular brown adipose tissue can be used as a measure of BAT thermogenesis. However, obtaining such measurements of sufficient quality is very difficult, and interpreting them can be tricky. Temperature differences between the inter-scapular and lumbar areas can be a better measure of the thermogenic activity of inter-scapular brown adipose tissue.Implanted probes for precise determination of BAT temperature (changes) should be fixed close to the Sulzer's vein. For measurement of BAT thermogenesis, core body temperature and BAT temperature should be recorded simultaneously.Tail temperature is suitable to compare the presence or absence of vasoconstriction or vasodilation.Continuous, longitudinal monitoring of core body temperature is preferred over single probing, as the readings are taken in a non-invasive, physiological context.Combining core body temperature measurements with metabolic rate measurements yields insights into the interplay between heat production and heat loss (thermal conductance), potentially revealing novel thermoregulatory phenotypes. Endothermic organisms rely on tightly balanced energy budgets to maintain a regulated body temperature and body mass. Metabolic phenotyping of mice, therefore, often includes the recording of body temperature. Thermometry in mice is conducted at various sites, using various devices and measurement practices, ranging from single-time probing to continuous temperature imaging. Whilst there is broad agreement that body temperature data is of value, procedural considerations of body temperature measurements in the context of metabolic phenotyping are missing. Here, we provide an overview of the various methods currently available for gathering body temperature data from mice. We explore the scope and limitations of thermometry in mice, with the hope of assisting researchers in the selection of appropriate approaches, and conditions, for comprehensive mouse phenotypic analyses.
Keywords: body temperature; metabolism; mouse; mouse models; phenotyping; telemetric recordings; thermography.
Figures



References
-
- Almeida M. C., Hew-Butler T., Soriano R. N., Rao S., Wang W., Wang J., et al. . (2012). Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J. Neurosci. 32, 2086–2099. 10.1523/JNEUROSCI.5606-11.2012 - DOI - PMC - PubMed
-
- Baldwin B. A. (1968). Behavioural thermoregulation in mice. Physiol. Behav. 3, 401–407. 10.1016/0031-9384(68)90069-3 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

