Intrinsic Plasma Cell Differentiation Defects in B Cell Expansion with NF-κB and T Cell Anergy Patient B Cells
- PMID: 28824638
- PMCID: PMC5539167
- DOI: 10.3389/fimmu.2017.00913
Intrinsic Plasma Cell Differentiation Defects in B Cell Expansion with NF-κB and T Cell Anergy Patient B Cells
Abstract
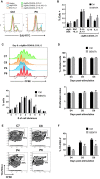
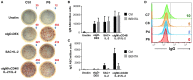
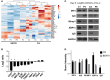
B cell Expansion with NF-κB and T cell Anergy (BENTA) disease is a novel B cell lymphoproliferative disorder caused by germline, gain-of-function mutations in the lymphocyte scaffolding protein CARD11, which drives constitutive NF-κB signaling. Despite dramatic polyclonal expansion of naive and immature B cells, BENTA patients also present with signs of primary immunodeficiency, including markedly reduced percentages of class-switched/memory B cells and poor humoral responses to certain vaccines. Using purified naive B cells from our BENTA patient cohort, here we show that BENTA B cells exhibit intrinsic defects in B cell differentiation. Despite a profound in vitro survival advantage relative to normal donor B cells, BENTA patient B cells were severely impaired in their ability to differentiate into short-lived IgDloCD38hi plasmablasts or CD138+ long-lived plasma cells in response to various stimuli. These defects corresponded with diminished IgG antibody production and correlated with poor induction of specific genes required for plasma cell commitment. These findings provide important mechanistic clues that help explain both B cell lymphocytosis and humoral immunodeficiency in BENTA disease.
Keywords: B cell Expansion with NF-κB and T cell Anergy; BLIMP-1; CARD11; antibodies; humans; plasma cells; primary immunodeficiency.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

