Voltage-Dependent Anion Channel 1 As an Emerging Drug Target for Novel Anti-Cancer Therapeutics
- PMID: 28824871
- PMCID: PMC5534932
- DOI: 10.3389/fonc.2017.00154
Voltage-Dependent Anion Channel 1 As an Emerging Drug Target for Novel Anti-Cancer Therapeutics
Abstract
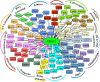
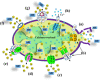
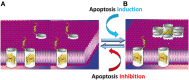
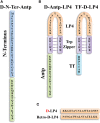
Cancer cells share several properties, high proliferation potential, reprogramed metabolism, and resistance to apoptotic cues. Acquiring these hallmarks involves changes in key oncogenes and non-oncogenes essential for cancer cell survival and prosperity, and is accompanied by the increased energy requirements of proliferating cells. Mitochondria occupy a central position in cell life and death with mitochondrial bioenergetics, biosynthesis, and signaling are critical for tumorigenesis. Voltage-dependent anion channel 1 (VDAC1) is situated in the outer mitochondrial membrane (OMM) and serving as a mitochondrial gatekeeper. VDAC1 allowing the transfer of metabolites, fatty acid ions, Ca2+, reactive oxygen species, and cholesterol across the OMM and is a key player in mitochondrial-mediate apoptosis. Moreover, VDAC1 serves as a hub protein, interacting with diverse sets of proteins from the cytosol, endoplasmic reticulum, and mitochondria that together regulate metabolic and signaling pathways. The observation that VDAC1 is over-expressed in many cancers suggests that the protein may play a pivotal role in cancer cell survival. However, VDAC1 is also important in mitochondria-mediated apoptosis, mediating release of apoptotic proteins and interacting with anti-apoptotic proteins, such as B-cell lymphoma 2 (Bcl-2), Bcl-xL, and hexokinase (HK), which are also highly expressed in many cancers. Strategically located in a "bottleneck" position, controlling metabolic homeostasis and apoptosis, VDAC1 thus represents an emerging target for anti-cancer drugs. This review presents an overview on the multi-functional mitochondrial protein VDAC1 performing several functions and interacting with distinct sets of partners to regulate both cell life and death, and highlights the importance of the protein for cancer cell survival. We address recent results related to the mechanisms of VDAC1-mediated apoptosis and the potential of associated proteins to modulate of VDAC1 activity, with the aim of developing VDAC1-based approaches. The first strategy involves modification of cell metabolism using VDAC1-specific small interfering RNA leading to inhibition of cancer cell and tumor growth and reversed oncogenic properties. The second strategy involves activation of cancer cell death using VDAC1-based peptides that prevent cell death induction by anti-apoptotic proteins. Finally, we discuss the potential therapeutic benefits of treatments and drugs leading to enhanced VDAC1 expression or targeting VDAC1 to induce apoptosis.
Keywords: apoptosis; cancer; metabolism; mitochondria; voltage-dependent anion channel 1.
Figures

Similar articles
-
The mitochondrial voltage-dependent anion channel 1 in tumor cells.Biochim Biophys Acta. 2015 Oct;1848(10 Pt B):2547-75. doi: 10.1016/j.bbamem.2014.10.040. Epub 2014 Nov 4. Biochim Biophys Acta. 2015. PMID: 25448878 Review.
-
VDAC1 at the crossroads of cell metabolism, apoptosis and cell stress.Cell Stress. 2017 Oct;1(1):11-36. doi: 10.15698/cst2017.10.104. Epub 2017 Oct 1. Cell Stress. 2017. PMID: 30542671 Free PMC article.
-
VDAC1-based peptides: novel pro-apoptotic agents and potential therapeutics for B-cell chronic lymphocytic leukemia.Cell Death Dis. 2013 Sep 19;4(9):e809. doi: 10.1038/cddis.2013.316. Cell Death Dis. 2013. PMID: 24052077 Free PMC article.
-
VDAC1 functions in Ca2+ homeostasis and cell life and death in health and disease.Cell Calcium. 2018 Jan;69:81-100. doi: 10.1016/j.ceca.2017.06.007. Epub 2017 Jun 23. Cell Calcium. 2018. PMID: 28712506 Review.
-
The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria.J Biol Chem. 2015 Apr 3;290(14):9150-61. doi: 10.1074/jbc.M114.622514. Epub 2015 Feb 13. J Biol Chem. 2015. PMID: 25681439 Free PMC article.
Cited by
-
The Interplay between Dysregulated Ion Transport and Mitochondrial Architecture as a Dangerous Liaison in Cancer.Int J Mol Sci. 2021 May 14;22(10):5209. doi: 10.3390/ijms22105209. Int J Mol Sci. 2021. PMID: 34069047 Free PMC article. Review.
-
A Mitochondrial VDAC1-Based Peptide Greatly Suppresses Steatosis and NASH-Associated Pathologies in a Mouse Model.Mol Ther. 2019 Oct 2;27(10):1848-1862. doi: 10.1016/j.ymthe.2019.06.017. Epub 2019 Jul 12. Mol Ther. 2019. PMID: 31375359 Free PMC article.
-
Metabolic Reprograming Via Silencing of Mitochondrial VDAC1 Expression Encourages Differentiation of Cancer Cells.Mol Ther Nucleic Acids. 2019 Sep 6;17:24-37. doi: 10.1016/j.omtn.2019.05.003. Epub 2019 May 18. Mol Ther Nucleic Acids. 2019. PMID: 31195298 Free PMC article.
-
Adverse Effects of Metformin From Diabetes to COVID-19, Cancer, Neurodegenerative Diseases, and Aging: Is VDAC1 a Common Target?Front Physiol. 2021 Oct 4;12:730048. doi: 10.3389/fphys.2021.730048. eCollection 2021. Front Physiol. 2021. PMID: 34671273 Free PMC article. Review.
-
Mechanisms of Cell Death Induced by Erastin in Human Ovarian Tumor Cells.Int J Mol Sci. 2024 Aug 8;25(16):8666. doi: 10.3390/ijms25168666. Int J Mol Sci. 2024. PMID: 39201357 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

