Prolonged human neural stem cell maturation supports recovery in injured rodent CNS
- PMID: 28825600
- PMCID: PMC5669577
- DOI: 10.1172/JCI92955
Prolonged human neural stem cell maturation supports recovery in injured rodent CNS
Abstract
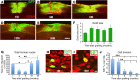
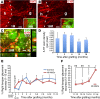
Neural stem cells (NSCs) differentiate into both neurons and glia, and strategies using human NSCs have the potential to restore function following spinal cord injury (SCI). However, the time period of maturation for human NSCs in adult injured CNS is not well defined, posing fundamental questions about the design and implementation of NSC-based therapies. This work assessed human H9 NSCs that were implanted into sites of SCI in immunodeficient rats over a period of 1.5 years. Notably, grafts showed evidence of continued maturation over the entire assessment period. Markers of neuronal maturity were first expressed 3 months after grafting. However, neurogenesis, neuronal pruning, and neuronal enlargement continued over the next year, while total graft size remained stable over time. Axons emerged early from grafts in very high numbers, and half of these projections persisted by 1.5 years. Mature astrocyte markers first appeared after 6 months, while more mature oligodendrocyte markers were not present until 1 year after grafting. Astrocytes slowly migrated from grafts. Notably, functional recovery began more than 1 year after grafting. Thus, human NSCs retain an intrinsic human rate of maturation, despite implantation into the injured rodent spinal cord, yet they support delayed functional recovery, a finding of great importance in planning human clinical trials.
Conflict of interest statement
Figures





Comment in
-
Patience pays in spinal repair.J Clin Invest. 2017 Sep 1;127(9):3284-3286. doi: 10.1172/JCI96650. Epub 2017 Aug 21. J Clin Invest. 2017. PMID: 28825601 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

