Sex-specific transcriptional signatures in human depression
- PMID: 28825715
- PMCID: PMC5734943
- DOI: 10.1038/nm.4386
Sex-specific transcriptional signatures in human depression
Erratum in
-
Corrigendum: Sex-specific transcriptional signatures in human depression.Nat Med. 2018 Apr 10;24(4):525. doi: 10.1038/nm0418-525d. Nat Med. 2018. PMID: 29634684
Abstract
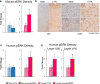
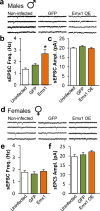
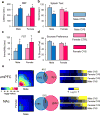
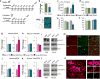
Major depressive disorder (MDD) is a leading cause of disease burden worldwide. While the incidence, symptoms and treatment of MDD all point toward major sex differences, the molecular mechanisms underlying this sexual dimorphism remain largely unknown. Here, combining differential expression and gene coexpression network analyses, we provide a comprehensive characterization of male and female transcriptional profiles associated with MDD across six brain regions. We overlap our human profiles with those from a mouse model, chronic variable stress, and capitalize on converging pathways to define molecular and physiological mechanisms underlying the expression of stress susceptibility in males and females. Our results show a major rearrangement of transcriptional patterns in MDD, with limited overlap between males and females, an effect seen in both depressed humans and stressed mice. We identify key regulators of sex-specific gene networks underlying MDD and confirm their sex-specific impact as mediators of stress susceptibility. For example, downregulation of the female-specific hub gene Dusp6 in mouse prefrontal cortex mimicked stress susceptibility in females, but not males, by increasing ERK signaling and pyramidal neuron excitability. Such Dusp6 downregulation also recapitulated the transcriptional remodeling that occurs in prefrontal cortex of depressed females. Together our findings reveal marked sexual dimorphism at the transcriptional level in MDD and highlight the importance of studying sex-specific treatments for this disorder.
Figures













Comment in
-
Sex-specific disease-associated modules for depression.Nat Med. 2017 Sep 8;23(9):1015-1017. doi: 10.1038/nm.4391. Nat Med. 2017. PMID: 28886002 Free PMC article.
References
-
- Organization., W. H. The Global burden of Disease 2004 update. 2008
-
- Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand. 2003;108:163–174. - PubMed
-
- Breslau N. Gender differences in trauma and posttraumatic stress disorder. The journal of gender-specific medicine: JGSM: the official journal of the Partnership for Women’s Health at Columbia. 2002;5:34–40. - PubMed
-
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. - PubMed
MeSH terms
Grants and funding
- F30 MH110073/MH/NIMH NIH HHS/United States
- R37 DA023206/DA/NIDA NIH HHS/United States
- R01 MH104559/MH/NIMH NIH HHS/United States
- R21 DA044538/DA/NIDA NIH HHS/United States
- R21 MH099562/MH/NIMH NIH HHS/United States
- S10 OD018522/OD/NIH HHS/United States
- T32 MH087004/MH/NIMH NIH HHS/United States
- R01 DA034856/DA/NIDA NIH HHS/United States
- R01 DA040620/DA/NIDA NIH HHS/United States
- P50 MH096890/MH/NIMH NIH HHS/United States
- R01 MH051399/MH/NIMH NIH HHS/United States
- R01 MH090264/MH/NIMH NIH HHS/United States
- T32 MH096678/MH/NIMH NIH HHS/United States
- F30 MH106293/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

