Regulation of life span by the gut microbiota in the short-lived African turquoise killifish
- PMID: 28826469
- PMCID: PMC5566455
- DOI: 10.7554/eLife.27014
Regulation of life span by the gut microbiota in the short-lived African turquoise killifish
Abstract
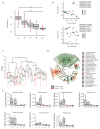
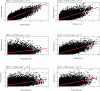
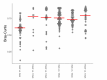
Gut bacteria occupy the interface between the organism and the external environment, contributing to homeostasis and disease. Yet, the causal role of the gut microbiota during host aging is largely unexplored. Here, using the African turquoise killifish (Nothobranchius furzeri), a naturally short-lived vertebrate, we show that the gut microbiota plays a key role in modulating vertebrate life span. Recolonizing the gut of middle-age individuals with bacteria from young donors resulted in life span extension and delayed behavioral decline. This intervention prevented the decrease in microbial diversity associated with host aging and maintained a young-like gut bacterial community, characterized by overrepresentation of the key genera Exiguobacterium, Planococcus, Propionigenium and Psychrobacter. Our findings demonstrate that the natural microbial gut community of young individuals can causally induce long-lasting beneficial systemic effects that lead to life span extension in a vertebrate model.
Keywords: Nothobranchius furzeri; Turquoise killifish; aging; evolutionary biology; genomics; infectious disease; life span; longevity; microbiology; microbiome.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures










References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

