Identification and dynamics of the human ZDHHC16-ZDHHC6 palmitoylation cascade
- PMID: 28826475
- PMCID: PMC5582869
- DOI: 10.7554/eLife.27826
Identification and dynamics of the human ZDHHC16-ZDHHC6 palmitoylation cascade
Abstract
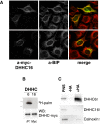
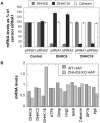
S-Palmitoylation is the only reversible post-translational lipid modification. Knowledge about the DHHC palmitoyltransferase family is still limited. Here we show that human ZDHHC6, which modifies key proteins of the endoplasmic reticulum, is controlled by an upstream palmitoyltransferase, ZDHHC16, revealing the first palmitoylation cascade. The combination of site specific mutagenesis of the three ZDHHC6 palmitoylation sites, experimental determination of kinetic parameters and data-driven mathematical modelling allowed us to obtain detailed information on the eight differentially palmitoylated ZDHHC6 species. We found that species rapidly interconvert through the action of ZDHHC16 and the Acyl Protein Thioesterase APT2, that each species varies in terms of turnover rate and activity, altogether allowing the cell to robustly tune its ZDHHC6 activity.
Keywords: Acyl protein thioesterase; DHHC; ERAD; cell biology; endoplasmic reticulum; human; mouse; palmitoylation; turnover.
Conflict of interest statement
No competing interests declared.
Figures








References
-
- Beard RS, Yang X, Meegan JE, Overstreet JW, Yang CG, Elliott JA, Reynolds JJ, Cha BJ, Pivetti CD, Mitchell DA, Wu MH, Deschenes RJ, Yuan SY. Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome. Nature Communications. 2016;7:12823. doi: 10.1038/ncomms12823. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

