Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition
- PMID: 28826492
- PMCID: PMC5589418
- DOI: 10.7554/eLife.27713
Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition
Abstract
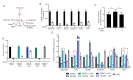
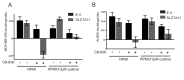
Many mammalian cancer cell lines depend on glutamine as a major tri-carboxylic acid (TCA) cycle anaplerotic substrate to support proliferation. However, some cell lines that depend on glutamine anaplerosis in culture rely less on glutamine catabolism to proliferate in vivo. We sought to understand the environmental differences that cause differential dependence on glutamine for anaplerosis. We find that cells cultured in adult bovine serum, which better reflects nutrients available to cells in vivo, exhibit decreased glutamine catabolism and reduced reliance on glutamine anaplerosis compared to cells cultured in standard tissue culture conditions. We find that levels of a single nutrient, cystine, accounts for the differential dependence on glutamine in these different environmental contexts. Further, we show that cystine levels dictate glutamine dependence via the cystine/glutamate antiporter xCT/SLC7A11. Thus, xCT/SLC7A11 expression, in conjunction with environmental cystine, is necessary and sufficient to increase glutamine catabolism, defining important determinants of glutamine anaplerosis and glutaminase dependence in cancer.
Keywords: SLC7A11; cancer; cancer biology; cystine; environment; glutaminase; human; metabolism; mouse.
Conflict of interest statement
No competing interests declared.
Is on the scientific advisory board of Agios Pharmaceuticals and Aeglea Biotherapeutics both of which seek to exploit altered metabolism for therapy.
Figures













References
-
- Baek S, Choi CM, Ahn SH, Lee JW, Gong G, Ryu JS, Oh SJ, Bacher-Stier C, Fels L, Koglin N, Hultsch C, Schatz CA, Dinkelborg LM, Mittra ES, Gambhir SS, Moon DH. Exploratory clinical trial of (4S)-4-(3-[18F]fluoropropyl)-L-glutamate for imaging xC- transporter using positron emission tomography in patients with non-small cell lung or breast cancer. Clinical Cancer Research. 2012;18:5427–5437. doi: 10.1158/1078-0432.CCR-12-0214. - DOI - PubMed
-
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV, Sonkin D, Reddy A, Liu M, Murray L, Berger MF, Monahan JE, Morais P, Meltzer J, Korejwa A, Jané-Valbuena J, Mapa FA, Thibault J, Bric-Furlong E, Raman P, Shipway A, Engels IH, Cheng J, Yu GK, Yu J, Aspesi P, de Silva M, Jagtap K, Jones MD, Wang L, Hatton C, Palescandolo E, Gupta S, Mahan S, Sougnez C, Onofrio RC, Liefeld T, MacConaill L, Winckler W, Reich M, Li N, Mesirov JP, Gabriel SB, Getz G, Ardlie K, Chan V, Myer VE, Weber BL, Porter J, Warmuth M, Finan P, Harris JL, Meyerson M, Golub TR, Morrissey MP, Sellers WR, Schlegel R, Garraway LA. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–307. doi: 10.1038/nature11003. - DOI - PMC - PubMed
-
- Biancur DE, Paulo JA, Małachowska B, Quiles Del Rey M, Sousa CM, Wang X, Sohn ASW, Chu GC, Gygi SP, Harper JW, Fendler W, Mancias JD, Kimmelman AC. Compensatory metabolic networks in pancreatic cancers upon perturbation of glutamine metabolism. Nature Communications. 2017;8:15965. doi: 10.1038/ncomms15965. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

