Small-molecule PET Tracers for Imaging Proteinopathies
- PMID: 28826526
- PMCID: PMC5657567
- DOI: 10.1053/j.semnuclmed.2017.06.003
Small-molecule PET Tracers for Imaging Proteinopathies
Abstract
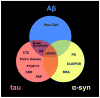
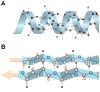
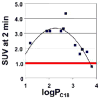
In this chapter, we provide a review of the challenges and advances in developing successful PET imaging agents for 3 major types of aggregated amyloid proteins: amyloid-beta (Aβ), tau, and alpha-synuclein (α-syn). These 3 amyloids are involved in the pathogenesis of a variety of neurodegenerative diseases, referred to as proteinopathies or proteopathies, that include Alzheimer disease, Lewy body dementias, multiple system atrophy, and frontotemporal dementias, among others. In the Introduction section, we briefly discuss the history of amyloid in neurodegenerative diseases and describe why progress in developing effective imaging agents has been hampered by the failure of crystallography to provide definitive ligand-protein interactions for rational radioligand design efforts. Instead, the field has relied on largely serendipitous, trial-and-error methods to achieve useful and specific PET amyloid imaging tracers for Aβ, tau, and α-syn deposits. Because many of the proteopathies involve more than 1 amyloid protein, it is important to develop selective PET tracers for the different amyloids to help assess the relative contribution of each to total amyloid burden. We use Pittsburgh compound B to illustrate some of the critical steps in developing a potent and selective Aβ PET imaging agent. Other selective Aβ and tau PET imaging compounds have followed similar pathways in their developmental processes. Success for selective α-syn PET imaging agents has not been realized yet, but work is ongoing in multiple laboratories throughout the world. In the tau sections, we provide background regarding 3-repeat (3R) and 4-repeat (4R) tau proteins and how they can affect the binding of tau radioligands in different tauopathies. We review the ongoing efforts to assess the properties of tau ligands, which are useful in 3R, 4R, or combined 3R-4R tauopathies. Finally, we describe in the α-syn sections recent attempts to develop selective tracers to image α-synucleinopathies.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


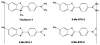
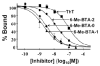
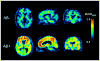
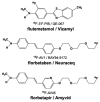


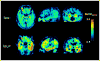


References
-
- Kumar V, Sami N, Kashav T, et al. Protein aggregation and neurodegenerative diseases: From theory to therapy. Eur J Med Chem. 2016;124:1105–1120. - PubMed
-
- Bourdenx M, Koulakiotis NS, Sanoudou D, et al. Protein aggregation and neurodegeneration in prototypical neurodegenerative diseases: Examples of amyloidopathies, tauopathies and synucleinopathies. Prog Neurobiol. 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

