Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial
- PMID: 28826616
- PMCID: PMC5718627
- DOI: 10.1016/S2352-3026(17)30140-0
Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial
Erratum in
-
Correction to Lancet Haematol 2017; 4: e431-42.Lancet Haematol. 2018 Aug;5(8):e332. doi: 10.1016/S2352-3026(18)30114-5. Epub 2018 Jul 18. Lancet Haematol. 2018. PMID: 30030067 No abstract available.
-
Correction to Lancet Haematol 2017; 4: e431-42.Lancet Haematol. 2018 Dec;5(12):e608. doi: 10.1016/S2352-3026(18)30199-6. Lancet Haematol. 2018. PMID: 30501866 No abstract available.
Abstract
Background: In the CALGB (Alliance) 100104 study, lenalidomide versus placebo after autologous stem-cell transplantation (ASCT) was investigated for patients with newly diagnosed myeloma. That study showed improved time to progression and overall survival and an increase in second primary malignancies for lenalidomide at a median follow-up of 34 months. Here we report an updated intention-to-treat analysis of CALGB (Alliance) 100104 at a median follow-up of 91 months.
Methods: Patients were eligible for this randomised, double-blind, placebo-controlled, phase 3 trial if they had symptomatic disease requiring treatment; had received, at most, two induction regimens; and had achieved stable disease or better in the first 100 days after ASCT. We randomly assigned patients to either lenalidomide or placebo groups using permuted block randomisation, with a fixed block size of six. Randomisation was stratified by three factors: normal or elevated β2 microglobulin concentration at registration (≤2·5 mg/L vs >2·5 mg/L), previous use or non-use of thalidomide during induction therapy, and previous use or non-use of lenalidomide during induction therapy. The starting dose was two capsules (10 mg) per day, escalated to three capsules (15 mg) per day after 3 months. The primary endpoint was time to progression (time of progressive disease or death from any cause), with intention-to-treat analysis. This study is registered with ClinicalTrials.gov, identifier NCT00114101. New patients are no longer being recruited, but some patients remain on treatment and in follow-up.
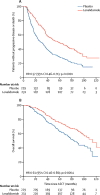
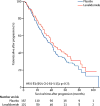
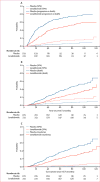
Findings: Between April 14, 2005, and July 2, 2009, 460 patients were randomly assigned to receive either lenalidomide (n=231) or placebo (n=229). After three interim analyses, the study was unblinded at a median follow-up of 18 months, at which point 86 (67%) of 128 patients without progressive disease in the placebo group chose to cross over to the lenalidomide group. The median follow-up for the updated survival analysis, as of Oct 19, 2016, was 91 months (IQR 83·6-103·1). The median time to progression was 57·3 months (95% CI 44·2-73·3) for the lenalidomide group and 28·9 months (23·0-36·3) for the placebo group (hazard ratio 0·57, 95% CI 0·46-0·71; p<0·0001). The most common grade 3-4 adverse events were neutropenia (116 [50%] patients in the lenalidomide group and 41 [18%] patients in the placebo group) and thrombocytopenia (34 [15%] patients in the lenalidomide group and 12 [5%] patients in the placebo group). 18 (8%) haematological and 14 (6%) solid tumour second primary malignancies were diagnosed after randomisation and before disease progression in the lenalidomide group, compared with three (1%) haematological and nine (4%) solid tumour second primary malignancies in the placebo group. Three haematological and five solid tumour second primary malignancies in the placebo group were in the crossover subgroup.
Interpretation: Despite an increase in haematological adverse events and second primary malignancies, lenalidomide maintenance therapy after ASCT significantly improved time to progression and could be considered a standard of care.
Funding: The National Cancer Institute.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Updated analysis of CALGB (Alliance) 100104.Lancet Haematol. 2017 Sep;4(9):e404-e405. doi: 10.1016/S2352-3026(17)30148-5. Epub 2017 Aug 17. Lancet Haematol. 2017. PMID: 28826617 No abstract available.
References
-
- Morgan GJ, Gregory WM, Davies FE, et al. The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012;119(1):7–15. - PubMed
-
- Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782–91. - PubMed
-
- Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;271(10):895–905. - PubMed
-
- Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide is a highly effective maintenance therapy in myeloma patients of all ages; results of the phase III Myeloma XI study. Blood (ASH Abstracts) 2016;128:1143.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA077658/CA/NCI NIH HHS/United States
- UG1 HL069286/HL/NHLBI NIH HHS/United States
- UG1 HL069249/HL/NHLBI NIH HHS/United States
- U01 HL069286/HL/NHLBI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 HL069294/HL/NHLBI NIH HHS/United States
- U10 CA032291/CA/NCI NIH HHS/United States
- UG1 HL069301/HL/NHLBI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U10 HL069286/HL/NHLBI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA007968/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 HL069301/HL/NHLBI NIH HHS/United States
- U10 CA021060/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 HL069315/HL/NHLBI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- U10 CA059518/CA/NCI NIH HHS/United States
- U10 CA077440/CA/NCI NIH HHS/United States
- UG1 HL109137/HL/NHLBI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- U10 CA037447/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- U10 CA047559/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- U01 HL069294/HL/NHLBI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
- U10 CA180799/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180838/CA/NCI NIH HHS/United States
- U10 CA180866/CA/NCI NIH HHS/United States
- U10 CA138561/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

