Phospholipase C2 Affects MAMP-Triggered Immunity by Modulating ROS Production
- PMID: 28827453
- PMCID: PMC5619888
- DOI: 10.1104/pp.17.00173
Phospholipase C2 Affects MAMP-Triggered Immunity by Modulating ROS Production
Abstract
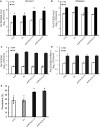
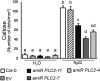
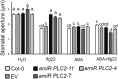
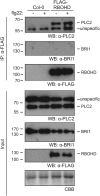
The activation of phosphoinositide-specific phospholipase C (PI-PLC) is one of the earliest responses triggered by the recognition of several microbe-associated molecular patterns (MAMPs) in plants. The Arabidopsis (Arabidopsis thaliana) PI-PLC gene family is composed of nine members. Previous studies suggested a role for PLC2 in MAMP-triggered immunity, as it is rapidly phosphorylated in vivo upon treatment with the bacterial MAMP flg22. Here, we analyzed the role of PLC2 in plant immunity using an artificial microRNA to silence PLC2 expression in Arabidopsis. We found that PLC2-silenced plants are more susceptible to the type III secretion system-deficient bacterial strain Pseudomonas syringae pv tomato (Pst) DC3000 hrcC- and to the nonadapted pea (Pisum sativum) powdery mildew Erysiphe pisi However, PLC2-silenced plants display normal susceptibility to virulent (Pst DC3000) and avirulent (Pst DC3000 AvrRPM1) P. syringae strains, conserving typical hypersensitive response features. In response to flg22, PLC2-silenced plants maintain wild-type mitogen-activated protein kinase activation and PHI1, WRKY33, and FRK1 immune marker gene expression but have reduced reactive oxygen species (ROS)-dependent responses such as callose deposition and stomatal closure. Accordingly, the generation of ROS upon flg22 treatment is compromised in the PLC2-defficient plants, suggesting an effect of PLC2 in a branch of MAMP-triggered immunity and nonhost resistance that involves early ROS-regulated processes. Consistently, PLC2 associates with the NADPH oxidase RBOHD, suggesting its potential regulation by PLC2.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Abd-El-Haliem AM, Vossen JH, van Zeijl A, Dezhsetan S, Testerink C, Seidl MF, Beck M, Strutt J, Robatzek S, Joosten MH (2016) Biochemical characterization of the tomato phosphatidylinositol-specific phospholipase C (PI-PLC) family and its role in plant immunity. Biochim Biophys Acta 1861: 1365–1378 - PubMed
-
- Andersson MX, Kourtchenko O, Dangl JL, Mackey D, Ellerström M (2006) Phospholipase-dependent signalling during the AvrRpm1- and AvrRpt2-induced disease resistance responses in Arabidopsis thaliana. Plant J 47: 947–959 - PubMed
-
- Anthony RG, Khan S, Costa J, Pais MS, Bögre L (2006) The Arabidopsis protein kinase PTI1-2 is activated by convergent phosphatidic acid and oxidative stress signaling pathways downstream of PDK1 and OXI1. J Biol Chem 281: 37536–37546 - PubMed
-
- Antolín-Llovera M, Petutsching EK, Ried MK, Lipka V, Nürnberger T, Robatzek S, Parniske M (2014) Knowing your friends and foes: plant receptor-like kinases as initiators of symbiosis or defence. New Phytol 204: 791–802 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

