Self-sorted Oligophenylvinylene and Perylene Bisimide Hydrogels
- PMID: 28827598
- PMCID: PMC5566499
- DOI: 10.1038/s41598-017-08644-0
Self-sorted Oligophenylvinylene and Perylene Bisimide Hydrogels
Abstract
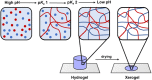
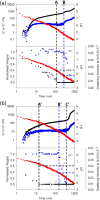
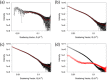
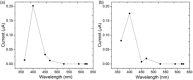
We describe two component hydrogels with networks composed of self-sorted fibres. The component gelators are based on 1,4-distyrylbenzene (OPV3) and perylene bisimide (PBI) units. Self-sorted gels can be formed by a slow decrease in pH, which leads to sequential assembly. We demonstrate self-sorting by NMR, rheology and small angle X-ray scattering (SAXS). Photoconductive xerogels can be prepared by drying these gels. The wavelength response of the xerogel is different to that of the PBI alone.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

