In vivo label-free structural and biochemical imaging of coronary arteries using an integrated ultrasound and multispectral fluorescence lifetime catheter system
- PMID: 28827758
- PMCID: PMC5566546
- DOI: 10.1038/s41598-017-08056-0
In vivo label-free structural and biochemical imaging of coronary arteries using an integrated ultrasound and multispectral fluorescence lifetime catheter system
Abstract
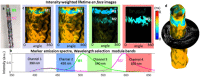
Existing clinical intravascular imaging modalities are not capable of accurate detection of critical plaque pathophysiology in the coronary arteries. This study reports the first intravascular catheter combining intravascular ultrasound (IVUS) with multispectral fluorescence lifetime imaging (FLIm) that enables label-free simultaneous assessment of morphological and biochemical features of coronary vessels in vivo. A 3.7 Fr catheter with a fiber-optic channel was constructed based on a 40 MHz clinical IVUS catheter. The ability to safely acquire co-registered FLIm-IVUS data in vivo using Dextran40 solution flushing was demonstrated in swine coronary arteries. FLIm parameters from the arterial wall were consistent with the emission of fluorophores present in healthy arterial wall (collagen, elastin). Additionally, structural and biochemical features from atherosclerotic lesions were acquired in ex vivo human coronary samples and corroborated with histological findings. Current results show that FLIm parameters linked to the amount of structural proteins (e.g. collagen, elastin) and lipids (e.g. foam cells, extracellular lipids) in the first 200 μm of the intima provide important biochemical information that can supplement IVUS data for a comprehensive assessment of plaques pathophysiology. The unique FLIm-IVUS system evaluated here has the potential to provide a comprehensive insight into atherosclerotic lesion formation, diagnostics and response to therapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

