Efficient generation of bispecific IgG antibodies by split intein mediated protein trans-splicing system
- PMID: 28827777
- PMCID: PMC5567192
- DOI: 10.1038/s41598-017-08641-3
Efficient generation of bispecific IgG antibodies by split intein mediated protein trans-splicing system
Abstract
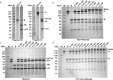
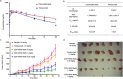
Many methods have been developed to produce bispecific antibodies (BsAbs) for industrial application. However, huge challenges still remain in synthesizing whole length BsAbs, including their assembly, stability, immunogenicity, and pharmacodynamics. Here we present for first time a generic technology platform of generating bispecific IgG antibodies, "Bispecific Antibody by Protein Trans-splicing (BAPTS)". Different from published methods, we assembled two parental antibody fragments in the hinge region by the protein trans-splicing reaction of a split intein to generate BsAbs without heavy/heavy and light/heavy chain mispairing. Utilizing this simple and efficient approach, there have been several BsAbs (CD3×HER2, CD3×EGFR, EGFR×HER2) synthesized to demonstrate its broad applicability. Correctly paired mAb arms were assembled to form BsAbs that were purified through protein A affinity chromatography to demonstrate industrial applicability at large scale. Further, the products were characterized through physical-biochemistry properties and biological activities to confirm expected quality of the products from "BAPTS". More importantly, correct pairing was confirmed by mass spectrum. Proof-of-concept studies with CD3×HER2 BsAb (T-cell recruitment) demonstrated superior bioactivity compared with trastuzumab. The results of undetectable mispairing and high biological activity have indicated that this method has the potential to be utilized to manufacture BsAbs with high efficiency at industrial scale.
Conflict of interest statement
Y.X., H.J., and J.Z. are employees of Jecho 566 Laboratories Inc.
Figures
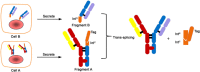



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

