mlh3 mutations in baker's yeast alter meiotic recombination outcomes by increasing noncrossover events genome-wide
- PMID: 28827832
- PMCID: PMC5578695
- DOI: 10.1371/journal.pgen.1006974
mlh3 mutations in baker's yeast alter meiotic recombination outcomes by increasing noncrossover events genome-wide
Erratum in
-
Correction: mlh3 mutations in baker's yeast alter meiotic recombination outcomes by increasing noncrossover events genome-wide.PLoS Genet. 2017 Oct 24;13(10):e1007067. doi: 10.1371/journal.pgen.1007067. eCollection 2017 Oct. PLoS Genet. 2017. PMID: 29065117 Free PMC article.
Abstract
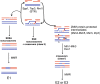
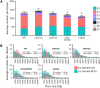
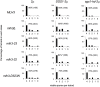
Mlh1-Mlh3 is an endonuclease hypothesized to act in meiosis to resolve double Holliday junctions into crossovers. It also plays a minor role in eukaryotic DNA mismatch repair (MMR). To understand how Mlh1-Mlh3 functions in both meiosis and MMR, we analyzed in baker's yeast 60 new mlh3 alleles. Five alleles specifically disrupted MMR, whereas one (mlh3-32) specifically disrupted meiotic crossing over. Mlh1-mlh3 representatives for each class were purified and characterized. Both Mlh1-mlh3-32 (MMR+, crossover-) and Mlh1-mlh3-45 (MMR-, crossover+) displayed wild-type endonuclease activities in vitro. Msh2-Msh3, an MSH complex that acts with Mlh1-Mlh3 in MMR, stimulated the endonuclease activity of Mlh1-mlh3-32 but not Mlh1-mlh3-45, suggesting that Mlh1-mlh3-45 is defective in MSH interactions. Whole genome recombination maps were constructed for wild-type and MMR+ crossover-, MMR- crossover+, endonuclease defective and null mlh3 mutants in an S288c/YJM789 hybrid background. Compared to wild-type, all of the mlh3 mutants showed increases in the number of noncrossover events, consistent with recombination intermediates being resolved through alternative recombination pathways. Our observations provide a structure-function map for Mlh3 that reveals the importance of protein-protein interactions in regulating Mlh1-Mlh3's enzymatic activity. They also illustrate how defective meiotic components can alter the fate of meiotic recombination intermediates, providing new insights for how meiotic recombination pathways are regulated.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Kunkel TA, Erie DA (2015) Eukaryotic Mismatch Repair in Relation to DNA Replication. Annu Rev Genet 49: 291–313. doi: 10.1146/annurev-genet-112414-054722 - DOI - PMC - PubMed
-
- Harfe BD, Minesinger BK, Jinks-Robertson S (2000) Discrete in vivo roles for the MutL homologs Mlh2p and Mlh3p in the removal of frameshift intermediates in budding yeast. Curr Biol 10: 145–148. - PubMed
-
- Romanova NV, Crouse GF (2013) Different roles of eukaryotic MutS and MutL complexes in repair of small insertion and deletion loops in yeast. PLoS Genet 9: e1003920 doi: 10.1371/journal.pgen.1003920 - DOI - PMC - PubMed
-
- Nishant KT, Plys AJ, Alani E (2008) A mutation in the putative MLH3 endonuclease domain confers a defect in both mismatch repair and meiosis in Saccharomyces cerevisiae. Genetics 179: 747–755. doi: 10.1534/genetics.108.086645 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

