Prediction of Intravenous Busulfan Clearance by Endogenous Plasma Biomarkers Using Global Pharmacometabolomics
- PMID: 28827982
- PMCID: PMC5562150
- DOI: 10.1007/s11306-016-1106-6
Prediction of Intravenous Busulfan Clearance by Endogenous Plasma Biomarkers Using Global Pharmacometabolomics
Abstract
Introduction: High-dose busulfan (busulfan) is an integral part of the majority of hematopoietic cell transplantation conditioning regimens. Intravenous (IV) busulfan doses are personalized using pharmacokinetics (PK)-based dosing where the patient's IV busulfan clearance is calculated after the first dose and is used to personalize subsequent doses to a target plasma exposure. PK-guided dosing has improved patient outcomes and is clinically accepted but highly resource intensive.
Objective: We sought to discover endogenous plasma biomarkers predictive of IV busulfan clearance using a global pharmacometabolomics-based approach.
Methods: Using LC-QTOF, we analyzed 59 (discovery) and 88 (validation) plasma samples obtained before IV busulfan administration.
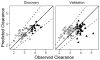
Results: In the discovery dataset, we evaluated the association of the relative abundance of 1885 ions with IV busulfan clearance and found 21 ions that were associated with IV busulfan clearance tertiles (r2 ≥ 0.3). Identified compounds were deoxycholic acid and/or chenodeoxycholic acid, and linoleic acid. We used these 21 ions to develop a parsimonious seven-ion linear predictive model that accurately predicted IV busulfan clearance in 93% (discovery) and 78% (validation) of samples.
Conclusion: IV busulfan clearance was significantly correlated with the relative abundance of 21 ions, seven of which were included in a predictive model that accurately predicted IV busulfan clearance in the majority of the validation samples. These results reinforce the potential of pharmacometabolomics as a critical tool in personalized medicine, with the potential to improve the personalized dosing of drugs with a narrow therapeutic index such as busulfan.
Keywords: biomarkers; busulfan; hematopoietic cell transplantation; personalized medicine; pharmacokinetics; pharmacometabolomics.
Conflict of interest statement
Conflict of Interest: All authors declare that they have no conflict of interest
Figures


References
-
- Administration, F. a. D. [Accessed October 18 2013];Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products — General Considerations. 2000 http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsar....
-
- Almog S, Kurnik D, Shimoni A, Loebstein R, Hassoun E, Gopher A, et al. Linearity and stability of intravenous busulfan pharmacokinetics and the role of glutathione in busulfan elimination. Biol Blood Marrow Transplant. 2011;17(1):117–123. doi: 10.1016/j.bbmt.2010.06.017. S1083-8791(10)00277-6 [pii] - DOI - PubMed
-
- Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36. JST.JSTAGE/dmpk/24.25 [pii] - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
