Sciatic nerve repair using poly(ε-caprolactone) tubular prosthesis associated with nanoparticles of carbon and graphene
- PMID: 28828216
- PMCID: PMC5561316
- DOI: 10.1002/brb3.755
Sciatic nerve repair using poly(ε-caprolactone) tubular prosthesis associated with nanoparticles of carbon and graphene
Abstract
Introduction: Injuries to peripheral nerves generate disconnection between spinal neurons and the target organ. Due to retraction of the nerve stumps, end-to-end neurorrhaphy is usually unfeasible. In such cases, autologous grafts are widely used, nonetheless with some disadvantages, such as mismatching of donor nerve dimensions and formation of painful neuromas at the donor area. Tubulization, using bioresorbable polymers, can potentially replace nerve grafting, although improvements are still necessary. Among promising bioresorbable synthetic polymers, poly(l-lactic acid) (PLLA) and poly(ε-caprolactone) (PCL) are the most studied. Carbon nanotubes and graphene sheets have been proposed, however, as adjuvants to improve mechanical and regenerative properties of tubular prostheses. Thus, the present work evaluated nerve tubulization repair following association of PCL with nanoparticles of carbon (NPC) and graphene (NPG).
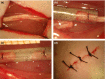
Methods: For that, adult Lewis rats were subjected to unilateral sciatic nerve tubulization and allowed to survive for up to 8 and 12 weeks postsurgery.
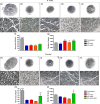
Results: Nanocomposites mechanical/chemical evaluation showed that nanoparticles do not alter PCL crystallinity, yet providing reinforcement of polymer matrix. Thus, there was a decrease in the enthalpy of melting when the mixture of PCL + NPC + NPG was used. Nanocomposites displayed positive changes in molecular mobility in the amorphous phase of the polymer. Also, the loss modulus (E") and the glass transition exhibited highest values for PCL + NPC + NPG. Scanning electron microscopy analysis revealed that PCL + NPC + NPG prostheses showed improved cell adhesion as compared to PCL alone. Surgical procedures with PCL + NPC + NPG were facilitated due to improved flexibility of the prosthesis, resulting in better stump positioning accuracy. In turn, a twofold increased number of myelinated axons was found in such repaired nerves. Consistent with that, target muscle atrophy protection has been observed.
Conclusion: Overall, the present data show that nanocomposite PCL tubes facilitate nerve repair and result in a better regenerative outcome, what may, in turn, represent a new alternative to pure PCL or PLLA prostheses.
Keywords: PCL; carbon nanotubes; graphene; peripheral nerves; tubulization.
Figures





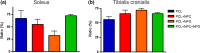
References
-
- Armentano, I. , Dottori, M. , Fortunati, E. , Mattioli, S. , & Kenny, J. M. (2010). Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polymer Degradation and Stability, 95, 2126–2146.
-
- Bergethon, P. R. , Trinkaus‐Randall, V. , & Franzblau, C. (1989). Modified hydroxyethylmethacrylate hydrogels as a modelling tool for the study of cell‐substratum interactions. Journal of Cell Science, 92(Pt 1), 111–121. - PubMed
-
- Bockelmann, J. , Klinkhammer, K. , von Holst, A. , Seiler, N. , Faissner, A. , Brook, G. A. , … Mey, J. (2011). Functionalization of electrospun poly(epsilon‐caprolactone) fibers with the extracellular matrix‐derived peptide GRGDS improves guidance of Schwann cell migration and axonal growth. Tissue Engineering Part A, 17, 475–486. - PubMed
-
- Brushart, T. M. , Mathur, V. , Sood, R. , & Koschorke, G. M. (1995). Joseph H. Boyes Award. Dispersion of regenerating axons across enclosed neural gaps. The Journal of Hand Surgery, 20, 557–564. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

