Evaluation of efficacy of metoprolol in patients having heart failure with preserved ejection fraction: A randomized, double-blind, placebo-controlled pilot trial
- PMID: 28828307
- PMCID: PMC5543763
- DOI: 10.4103/2229-3485.210449
Evaluation of efficacy of metoprolol in patients having heart failure with preserved ejection fraction: A randomized, double-blind, placebo-controlled pilot trial
Abstract
Background: There is a lack of evidence-based therapies for the treatment of heart failure (HF) with preserved ejection fraction (HFpEF). Beta blockers may provide some benefit in HFpEF due to their proven role in HF with reduced ejection fraction.
Aim: The main objective of the present study was to evaluate the efficacy of controlled-release metoprolol (metoprolol succinate) in HFpEF.
Materials and methods: This was an investigator-initiated, randomized, double-blind, placebo-controlled, 14-week pilot study with metoprolol succinate as a study drug. Dose titration protocol was used with optional upward titration of doses ranging from 25 to 100 mg. The end points included clinical, echocardiographic, biochemical (N-terminal pro-B-type natriuretic peptide and serum carboxy-terminal propeptide of procollagen type I), and quality of life (QoL) (SF-36) parameters.
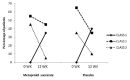
Results: Twenty patients were enrolled in each of the treatment arms. An improvement in New York Heart Association class and exercise capacity was seen in both treatment arms. The mean change in various echocardiographic and biochemical parameters between the two groups was statistically insignificant. A significant improvement in some QoL parameters was observed in both the groups. No serious adverse events were seen.
Conclusion: Hence, this pilot study showed that metoprolol succinate possibly has some beneficial role in HFpEF as reflected by improvement in some parameters. The findings highlight the need of a larger study with longer follow-up to provide a definitive answer.
Keywords: Diastolic dysfunction; heart failure; metoprolol; tissue Doppler; treadmill test.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Abbate A, Arena R, Abouzaki N, Van Tassell BW, Canada J, Shah K, et al. Heart failure with preserved ejection fraction: Refocusing on diastole. Int J Cardiol. 2015;179:430–40. - PubMed
-
- Nativi-Nicolau J, Ryan JJ, Fang JC. Current therapeutic approach in heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:525–38. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. - PubMed
-
- Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-preserved trial. Lancet. 2003;362:777–81. - PubMed
-
- Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous