Challenges in Development of Sperm Repositories for Biomedical Fishes: Quality Control in Small-Bodied Species
- PMID: 28829251
- PMCID: PMC5706620
- DOI: 10.1089/zeb.2017.1426
Challenges in Development of Sperm Repositories for Biomedical Fishes: Quality Control in Small-Bodied Species
Abstract
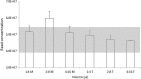
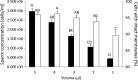
Quality control (QC) is essential for reproducible and efficient functioning of germplasm repositories. However, many biomedical fish models present significant QC challenges due to small body sizes (<5 cm) and miniscule sperm volumes (<5 μL). Using minimal volumes of sperm, we used Zebrafish to evaluate common QC endpoints as surrogates for fertilization success along sequential steps of cryopreservation. First, concentrations of calibration bead suspensions were evaluated with a Makler® counting chamber by using different sample volumes and mixing methods. For sperm analysis, samples were initially diluted at a 1:30 ratio with Hanks' balanced salt solution (HBSS). Motility was evaluated by using different ratios of sperm and activation medium, and membrane integrity was analyzed with flow cytometry at different concentrations. Concentration and sperm motility could be confidently estimated by using volumes as small as 1 μL, whereas membrane integrity required a minimum of 2 μL (at 1 × 106 cells/mL). Thus, <5 μL of sperm suspension (after dilution to 30-150 μL with HBSS) was required to evaluate sperm quality by using three endpoints. Sperm quality assessment using a combination of complementary endpoints enhances QC efforts during cryopreservation, increasing reliability and reproducibility, and reducing waste of time and resources.
Keywords: biomedical model fishes; quality control; reproducibility; sperm volume.
Conflict of interest statement
No competing financial interests exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

