Effect of Ganciclovir on IL-6 Levels Among Cytomegalovirus-Seropositive Adults With Critical Illness: A Randomized Clinical Trial
- PMID: 28829877
- PMCID: PMC5817487
- DOI: 10.1001/jama.2017.10569
Effect of Ganciclovir on IL-6 Levels Among Cytomegalovirus-Seropositive Adults With Critical Illness: A Randomized Clinical Trial
Abstract
Importance: The role of cytomegalovirus (CMV) reactivation in mediating adverse clinical outcomes in nonimmunosuppressed adults with critical illness is unknown.
Objective: To determine whether ganciclovir prophylaxis reduces plasma interleukin 6 (IL-6) levels in CMV-seropositive adults who are critically ill.
Design, setting, and participants: Double-blind, placebo-controlled, randomized clinical trial (conducted March 10, 2011-April 29, 2016) with a follow-up of 180 days (November 10, 2016) that included 160 CMV-seropositive adults with either sepsis or trauma and respiratory failure at 14 university intensive care units (ICUs) across the United States.
Interventions: Patients were randomized (1:1) to receive either intravenous ganciclovir (5 mg/kg twice daily for 5 days), followed by either intravenous ganciclovir or oral valganciclovir once daily until hospital discharge (n = 84) or to receive matching placebo (n = 76).
Main outcomes and measures: The primary outcome was change in IL-6 level from day 1 to 14. Secondary outcomes were incidence of CMV reactivation in plasma, mechanical ventilation days, incidence of secondary bacteremia or fungemia, ICU length of stay, mortality, and ventilator-free days (VFDs) at 28 days.
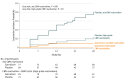
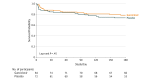
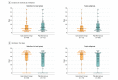
Results: Among 160 randomized patients (mean age, 57 years; women, 43%), 156 patients received 1or more dose(s) of study medication, and 132 patients (85%) completed the study. The mean change in plasma IL-6 levels between groups was -0.79 log10 units (-2.06 to 0.48) in the ganciclovir group and -0.79 log10 units (-2.14 to 0.56) in the placebo group (point estimate of difference, 0 [95% CI, -0.3 to 0.3]; P > .99). Among secondary outcomes, CMV reactivation in plasma was significantly lower in the ganciclovir group (12% [10 of 84 patients] vs 39% [28 of 72 patients]); absolute risk difference, -27 (95% CI, -40 to -14), P < .001. The ganciclovir group had more median VFDs in both the intention-to-treat (ITT) group and in the prespecified sepsis subgroup (ITT group: 23 days in ganciclovir group vs 20 days in the placebo group, P = .05; sepsis subgroup, 23 days in the ganciclovir group vs 20 days in the placebo group, P = .03). There were no significant differences between the ganciclovir and placebo groups in duration of mechanical ventilation (5 days for the ganciclovir group vs 6 days for the placebo group, P = .16), incidence of secondary bacteremia or fungemia (15% for the ganciclovir group vs 15% for the placebo group, P = .67), ICU length of stay (8 days for the ganciclovir group vs 8 days for the placebo group, P = .76), or mortality (12% for the ganciclovir group vs 15% for the placebo group, P = .54).
Conclusions and relevance: Among CMV-seropositive adults with critical illness due to sepsis or trauma, ganciclovir did not reduce IL-6 levels and the current study does not support routine clinical use of ganciclovir as a prophylactic agent in patients with sepsis. Additional research is necessary to determine the clinical efficacy and safety of CMV suppression in this setting.
Trial registration: clinicaltrials.gov Identifier: NCT01335932.
Conflict of interest statement
Figures

Comment in
-
Time to Consider Cytomegalovirus Prevention in Critically Ill Patients?JAMA. 2017 Aug 22;318(8):709-710. doi: 10.1001/jama.2017.10132. JAMA. 2017. PMID: 28829852 No abstract available.
References
-
- Toorkey CB, Carrigan DR. Immunohistochemical detection of an immediate early antigen of human cytomegalovirus in normal tissues. J Infect Dis. 1989;160(5):741-751. - PubMed
-
- Griffiths P, Baraniak I, Reeves M. The pathogenesis of human cytomegalovirus. J Pathol. 2015;235(2):288-297. - PubMed
-
- Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med. 2009;37(8):2350-2358. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

