Humanization of JAA-F11, a Highly Specific Anti-Thomsen-Friedenreich Pancarcinoma Antibody and InVitro Efficacy Analysis
- PMID: 28830009
- PMCID: PMC5565633
- DOI: 10.1016/j.neo.2017.07.001
Humanization of JAA-F11, a Highly Specific Anti-Thomsen-Friedenreich Pancarcinoma Antibody and InVitro Efficacy Analysis
Erratum in
-
Corrigendum to "Humanization of JAA-F11, a Highly Specific Anti-Thomsen-Friedenreich Pancarcinoma Antibody and In Vitro Efficacy Analysis" [Neoplasia 19.9 (2017) 716-733].Neoplasia. 2018 Jan;20(1):118. doi: 10.1016/j.neo.2017.10.001. Neoplasia. 2018. PMID: 29277397 Free PMC article. No abstract available.
Abstract
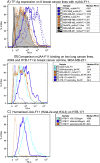
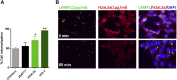
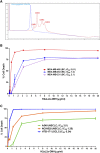
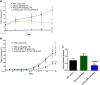
JAA-F11 is a highly specific mouse monoclonal to the Thomsen-Friedenreich Antigen (TF-Ag) which is an alpha-O-linked disaccharide antigen on the surface of ~80% of human carcinomas, including breast, lung, colon, bladder, ovarian, and prostate cancers, and is cryptic on normal cells. JAA-F11 has potential, when humanized, for cancer immunotherapy for multiple cancer types. Humanization of JAA-F11, was performed utilizing complementarity determining regions grafting on a homology framework. The objective herein is to test the specificity, affinity and biology efficacy of the humanized JAA-F11 (hJAA-F11). Using a 609 target glycan array, 2 hJAA-F11 constructs were shown to have excellent chemical specificity, binding only to TF-Ag alpha-linked structures and not to TF-Ag beta-linked structures. The relative affinity of these hJAA-F11 constructs for TF-Ag was improved over the mouse antibody, while T20 scoring predicted low clinical immunogenicity. The hJAA-F11 constructs produced antibody-dependent cellular cytotoxicity in breast and lung tumor lines shown to express TF-Ag by flow cytometry. Internalization of hJAA-F11 into cancer cells was also shown using a surface binding ELISA and confirmed by immunofluorescence microscopy. Both the naked hJAA-F11 and a maytansine-conjugated antibody (hJAA-F11-DM1) suppressed in vivo tumor progression in a human breast cancer xenograft model in SCID mice. Together, our results support the conclusion that the humanized antibody to the TF-Ag has potential as an adjunct therapy, either directly or as part of an antibody drug conjugate, to treat breast cancer, including triple negative breast cancer which currently has no targeted therapy, as well as lung cancer.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224(4654):1198–1206. - PubMed
-
- Springer GF, Murthy MS, Desai PR, Scanlon EF. Breast cancer patient's cell-mediated immune response to Thomsen-Friedenreich (T) antigen. Cancer. 1980;45:2949–2954. - PubMed
-
- Wolf M, Ludwig A, Fritz P, Schumacher K. Increased expression of Thomsen-Friedenreich antigens during tumor progression in breast cancer patients. Tumor Biol. 1988;9(4):190–194. - PubMed
-
- Takanami I. Expression of Thomsen-Friedenreich antigen as a marker of poor prognosis in pulmonary adenocarcinoma. Oncol Rep. 1999;6(2):341–345. - PubMed
-
- Cao Y, Karsten UR, Liebrich W, Haensch W, Springer GF, Schlag PM. Expression of thomsen-friedenreich-related antigens in primary and metastatic colorectal carcinomas. A reevaluation. Cancer. 1995;76(10):1700–1708. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

