Physiology, ecology and industrial applications of aroma formation in yeast
- PMID: 28830094
- PMCID: PMC5916228
- DOI: 10.1093/femsre/fux031
Physiology, ecology and industrial applications of aroma formation in yeast
Abstract
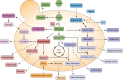
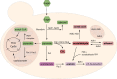
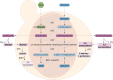
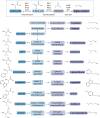
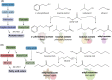
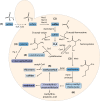
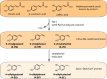
Yeast cells are often employed in industrial fermentation processes for their ability to efficiently convert relatively high concentrations of sugars into ethanol and carbon dioxide. Additionally, fermenting yeast cells produce a wide range of other compounds, including various higher alcohols, carbonyl compounds, phenolic compounds, fatty acid derivatives and sulfur compounds. Interestingly, many of these secondary metabolites are volatile and have pungent aromas that are often vital for product quality. In this review, we summarize the different biochemical pathways underlying aroma production in yeast as well as the relevance of these compounds for industrial applications and the factors that influence their production during fermentation. Additionally, we discuss the different physiological and ecological roles of aroma-active metabolites, including recent findings that point at their role as signaling molecules and attractants for insect vectors.
© FEMS 2017. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Engineering the cytosolic NADH availability in lager yeast to improve the aroma profile of beer.Biotechnol Lett. 2019 Mar;41(3):363-369. doi: 10.1007/s10529-019-02653-x. Epub 2019 Feb 1. Biotechnol Lett. 2019. PMID: 30707389
-
Wine flavor and aroma.J Ind Microbiol Biotechnol. 2011 Sep;38(9):1145-59. doi: 10.1007/s10295-011-1018-4. Epub 2011 Jul 24. J Ind Microbiol Biotechnol. 2011. PMID: 21786136 Review.
-
Nitrogenous Compound Utilization and Production of Volatile Organic Compounds among Commercial Wine Yeasts Highlight Strain-Specific Metabolic Diversity.Microbiol Spectr. 2021 Sep 3;9(1):e0048521. doi: 10.1128/Spectrum.00485-21. Epub 2021 Jul 21. Microbiol Spectr. 2021. PMID: 34287034 Free PMC article.
-
Linking gene regulation and the exo-metabolome: a comparative transcriptomics approach to identify genes that impact on the production of volatile aroma compounds in yeast.BMC Genomics. 2008 Nov 7;9:530. doi: 10.1186/1471-2164-9-530. BMC Genomics. 2008. PMID: 18990252 Free PMC article.
-
Engineering Saccharomyces cerevisiae for direct conversion of raw, uncooked or granular starch to ethanol.Crit Rev Biotechnol. 2015;35(3):369-91. doi: 10.3109/07388551.2014.888048. Crit Rev Biotechnol. 2015. PMID: 24666118 Review.
Cited by
-
The Altitude of Coffee Cultivation Causes Shifts in the Microbial Community Assembly and Biochemical Compounds in Natural Induced Anaerobic Fermentations.Front Microbiol. 2021 May 20;12:671395. doi: 10.3389/fmicb.2021.671395. eCollection 2021. Front Microbiol. 2021. PMID: 34093490 Free PMC article.
-
Nature's Most Fruitful Threesome: The Relationship between Yeasts, Insects, and Angiosperms.J Fungi (Basel). 2022 Sep 20;8(10):984. doi: 10.3390/jof8100984. J Fungi (Basel). 2022. PMID: 36294549 Free PMC article. Review.
-
Signaling by AWC Olfactory Neurons Is Necessary for Caenorhabditis elegans' Response to Prenol, an Odor Associated with Nematode-Infected Insects.Genetics. 2020 Sep;216(1):145-157. doi: 10.1534/genetics.120.303280. Epub 2020 Jul 17. Genetics. 2020. PMID: 32680884 Free PMC article.
-
Assessment of Tannin Tolerant Non-Saccharomyces Yeasts Isolated from Miang for Production of Health-Targeted Beverage Using Miang Processing Byproducts.J Fungi (Basel). 2023 Jan 27;9(2):165. doi: 10.3390/jof9020165. J Fungi (Basel). 2023. PMID: 36836280 Free PMC article.
-
Effects of Plant-Derived Polyphenols on the Antioxidant Activity and Aroma of Sulfur-Dioxide-Free Red Wine.Molecules. 2023 Jul 6;28(13):5255. doi: 10.3390/molecules28135255. Molecules. 2023. PMID: 37446916 Free PMC article.
References
-
- Adams MR, Moss MO. Food Microbiology. Cambridge, UK: Royal Society of Chemistry, 1995.
-
- Agarwal PK, Uppada V, Noronha SB. Comparison of pyruvate decarboxylases from Saccharomyces cerevisiae and Komagataella pastoris (Pichia pastoris). Appl Microbiol Biot 2013;97:9439–49. - PubMed
-
- Agrimi G, Mena MC, Izumi K et al. . Improved sake metabolic profile during fermentation due to increased mitochondrial pyruvate dissimilation. FEMS Yeast Res 2014;14:249–60. - PubMed
-
- Alba-Lois L, Segal-Kischinevzky C. Beer & Wine Makers. Nat Educ 2010;3:17.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

