Photosynthetic limitations in two Antarctic vascular plants: importance of leaf anatomical traits and Rubisco kinetic parameters
- PMID: 28830100
- PMCID: PMC5854023
- DOI: 10.1093/jxb/erx148
Photosynthetic limitations in two Antarctic vascular plants: importance of leaf anatomical traits and Rubisco kinetic parameters
Abstract
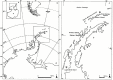
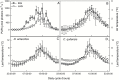
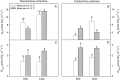
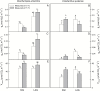
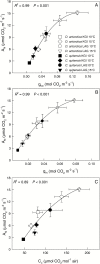
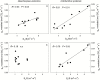
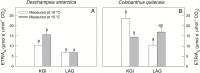
Particular physiological traits allow the vascular plants Deschampsia antarctica Desv. and Colobanthus quitensis (Kunth) Bartl. to inhabit Antarctica. The photosynthetic performance of these species was evaluated in situ, focusing on diffusive and biochemical constraints to CO2 assimilation. Leaf gas exchange, Chl a fluorescence, leaf ultrastructure, and Rubisco catalytic properties were examined in plants growing on King George and Lagotellerie islands. In spite of the species- and population-specific effects of the measurement temperature on the main photosynthetic parameters, CO2 assimilation was highly limited by CO2 diffusion. In particular, the mesophyll conductance (gm)-estimated from both gas exchange and leaf chlorophyll fluorescence and modeled from leaf anatomy-was remarkably low, restricting CO2 diffusion and imposing the strongest constraint to CO2 acquisition. Rubisco presented a high specificity for CO2 as determined in vitro, suggesting a tight co-ordination between CO2 diffusion and leaf biochemistry that may be critical ultimately to optimize carbon balance in these species. Interestingly, both anatomical and biochemical traits resembled those described in plants from arid environments, providing a new insight into plant functional acclimation to extreme conditions. Understanding what actually limits photosynthesis in these species is important to anticipate their responses to the ongoing and predicted rapid warming in the Antarctic Peninsula.
Keywords: Antarctic plants; Rubisco; leaf traits; mesophyll conductance; photosynthesis; temperature.
© The Author 2017. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures
References
-
- Alberdi M, Bravo LA, Gutiérrez A, Gidekel M, Corcuera LJ. 2002. Ecophysiology of Antarctic vascular plants. Physiologia Plantarum 115, 479–486. - PubMed
-
- Badger MR, Collatz GJ. 1977. Studies on the kinetic mechanism of ribulose-1, 5-bisphosphate carboxylase and oxygenase reactions, with particular reference to the effect of temperature on kinetic parameters. Carnegie Institute of Washington Yearbook 76, 355–361.
-
- Brooks A, Farquhar GD. 1985. Effect of temperature on the CO2/O2 specificity of ribulose-1, 5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165, 397–406. - PubMed
-
- Carriquí M, Cabrera HM, Conesa MÀ, et al. 2015. Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant, Cell and Environment 38, 448–460. - PubMed
-
- Cavieres LA, Sáez P, Sanhueza C, Sierra-Almeida A, Rabert C, Corcuera LJ, Bravo LA. 2016. Ecophysiological traits of Antarctic vascular plants: their importance in the responses to climate change. Plant Ecology 217, 343–358.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

