Hydrophilic intraocular lens opacification after posterior lamellar keratoplasty - a material analysis with special reference to optical quality assessment
- PMID: 28830376
- PMCID: PMC5568293
- DOI: 10.1186/s12886-017-0546-8
Hydrophilic intraocular lens opacification after posterior lamellar keratoplasty - a material analysis with special reference to optical quality assessment
Abstract
Background: Laboratory analysis and optical quality assessment of explanted hydrophilic intraocular lenses (IOLs) with clinically significant opacification after posterior lamellar keratoplasty (DMEK and DSAEK).
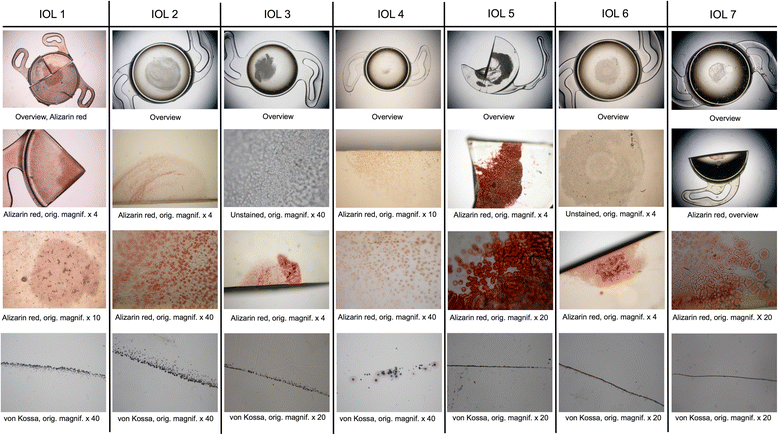
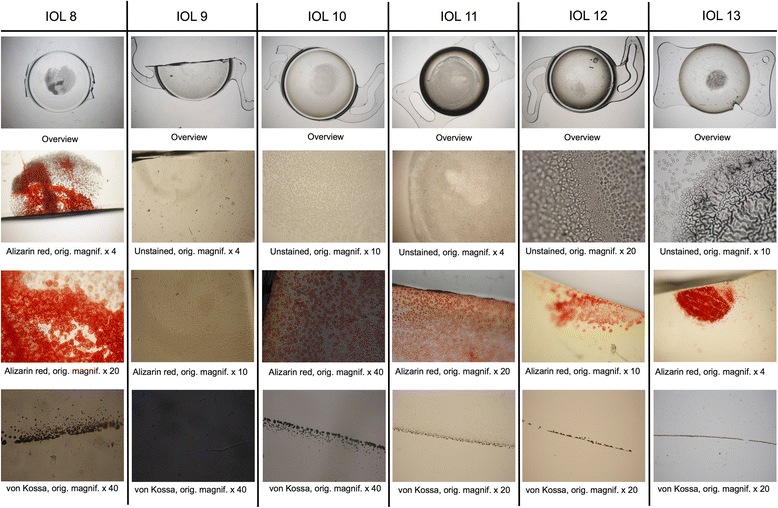
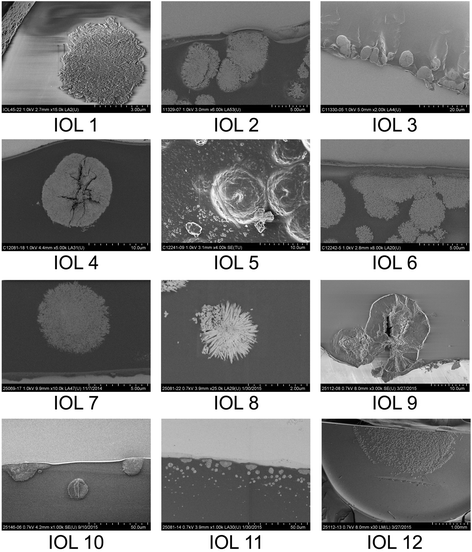
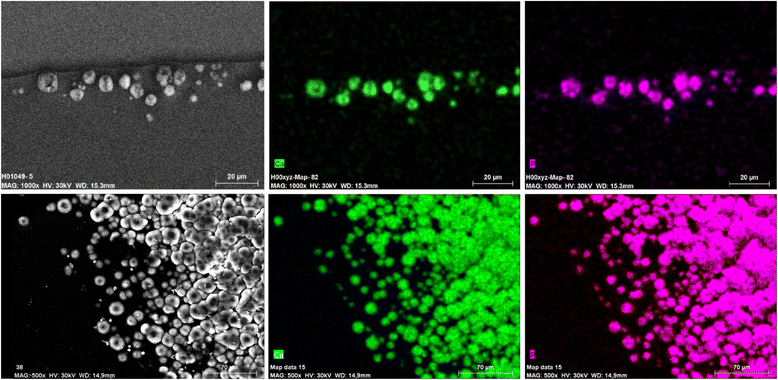
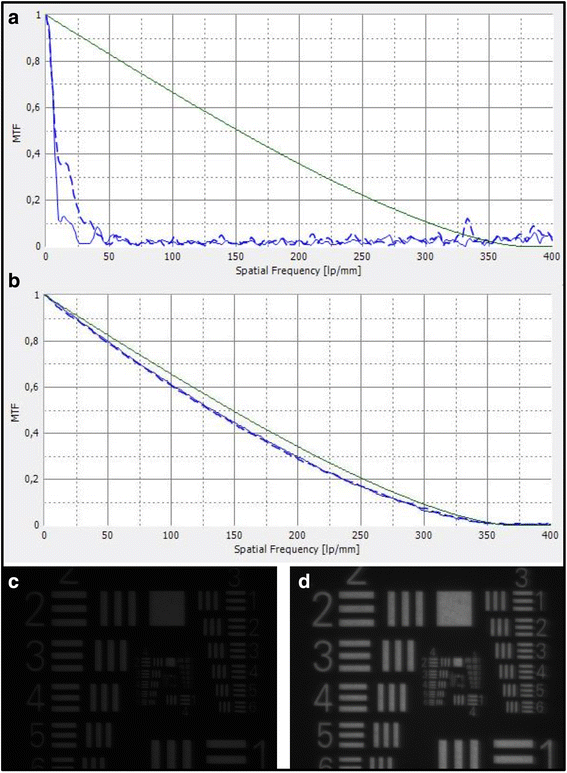
Methods: Thirteen opacified IOLs after posterior lamellar keratoplasty, 8 after descemet stripping automated endothelial keratoplasty (DSAEK), 3 after descemet membrane endothelial keratoplasty (DMEK) and 2 after both DSAEK and DMEK were analysed in our laboratory. Analyses included optical bench assessment for optical quality, light microscopy, scanning electron microscopy (SEM) and energy dispersive X-Ray spectroscopy (EDS).
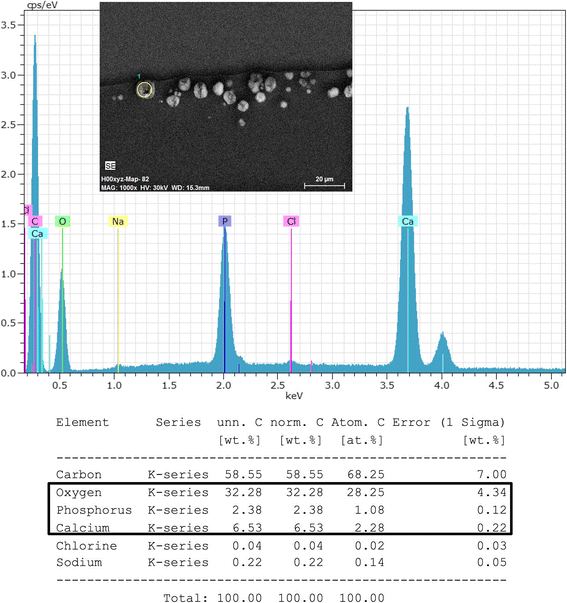
Results: In all IOLs the opacification was caused by a thin layer of calciumphosphate that had accumulated underneath the anterior optical surface of the IOLs in the area spared by the pupil/anterior capsulorhexis. The calcifications lead to a significant deterioration of the modulation transfer function across all spatial frequencies of the affected IOLs.
Conclusions: The instillation of exogenous material such as air or gas into the anterior chamber increases the risk for opacification of hydrophilic IOLs irrespective of the manufacturer or the exact composition of the hydrophilic lens material. It is recommended to avoid the use of hydrophilic acrylic IOLs in patients with endothelial dystrophy that will likely require procedures involving the intracameral instillation of air or gas, such as DMEK or DS(A)EK.
Keywords: Cataract surgery; Complications; Cornea; Descemet membrane endothelial keratoplasty; Fuchs endothelial dystrophy; IOL explantation; Intraocular lens; Optical quality; Posterior lamellar keratoplasty.
Conflict of interest statement
Ethics approval and consent to participate
This study solely involves laboratory analyses of IOL explants. No additional procedures on humans or animals were performed. An informed consent and ethics committee approval were therefore not required.
Consent for publication
Not applicable.
Competing interests
None of the authors has a financial or proprietary interest in any material or method mentioned.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Fung SS, Sykakis E, Islam NM, et al. Intraocular Lens Opacification following Intracameral Injection of Recombinant Tissue Plasminogen Activator to Treat Inflammatory Membranes after Cataract Surgery. J Ophthalmol. 2015;2015:975075 doi:10.1155/2015/975075 [published Online First: Epub Date]|. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

