Assessment of ibrutinib plus rituximab in front-line CLL (FLAIR trial): study protocol for a phase III randomised controlled trial
- PMID: 28830517
- PMCID: PMC5568356
- DOI: 10.1186/s13063-017-2138-6
Assessment of ibrutinib plus rituximab in front-line CLL (FLAIR trial): study protocol for a phase III randomised controlled trial
Abstract
Background: Treatment of chronic lymphocytic leukaemia (CLL) has seen a substantial improvement over the last few years. Combination immunochemotherapy, such as fludarabine, cyclophosphamide and rituximab (FCR), is now standard first-line therapy. However, the majority of patients relapse and require further therapy, and so new, effective, targeted therapies that improve remission rates, reduce relapses, and have fewer side effects, are required. The FLAIR trial will assess whether ibrutinib plus rituximab (IR) is superior to FCR in terms of progression-free survival (PFS).
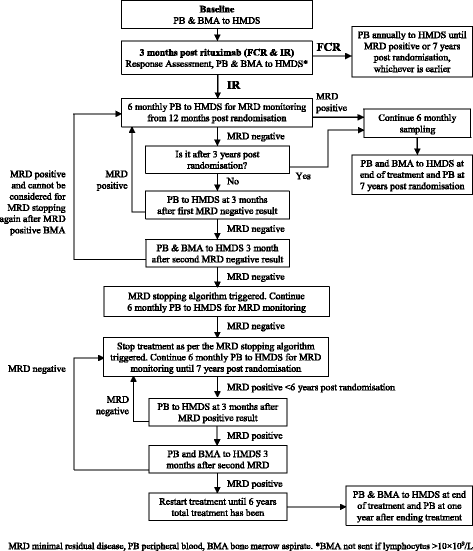
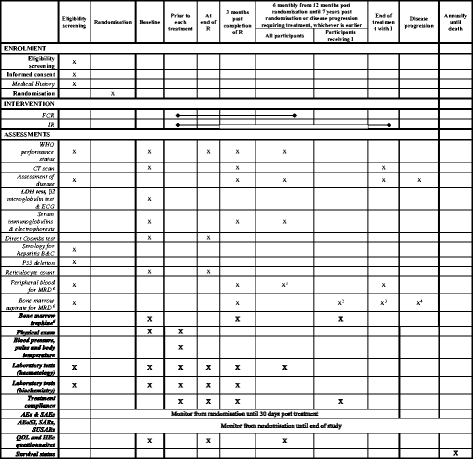
Methods/design: FLAIR is a phase III, multicentre, randomised, controlled, open, parallel-group trial in patients with previously untreated CLL. A total of 754 participants will be randomised on a 1:1 basis to receive standard therapy with FCR or IR. Participants randomised to FCR will receive a maximum of six 28-day treatment cycles. Participants randomised to IR will receive six 28-day cycles of rituximab, and ibrutinib taken daily for 6 years until minimal residual disease (MRD) negativity has been recorded for the same amount of time as it took to become MRD negative, or until disease progression. The primary endpoint is PFS according to the International Workshop on CLL (IWCLL) criteria. Secondary endpoints include: overall survival; proportion of participants with undetectable MRD; response to therapy by IWCLL criteria; safety and toxicity; health-related quality of life (QoL); and cost-effectiveness.
Discussion: The trial aims to provide evidence for the future first-line treatment of CLL patients by assessing whether IR is superior to FCR in terms of PFS, and whether toxicity rates are favourable.
Trial registration: ISRCTN01844152 . Registered on 8 August 2014, EudraCT number 2013-001944-76 . Registered on 26 April 2013.
Keywords: CLL; Clinical trial; FCR; Front-line; Ibrutinib; Minimal residual disease (MRD); Phase III; Randomised; Rituximab.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval has been obtained from the National Research Ethics Service Committee Yorkshire and Humber – Leeds East (reference 14/YH/0085), including approval to perform the study at all centres. A list of centres is provided in Additional file 2. All participants will give written informed consent before taking part.
Consent for publication
Not applicable.
Competing interests
PH has received research support and honoraria for lecturing from Roche, Janssen, Gilead, Abbvie and Novartis. AR has honoraria from Abbvie, Celgene, Gilead, GSK, Guidepoint Global, Janssen and Roche. The other authors declare that they have no competing interests
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Haematology Malignancy Research Network. Available from: https://www.hmrn.org/statistics/disorders/19. Accessed 12 Apr 2013.
-
- Advani R, Sharman J, Smith S. The BTK inhibitor PCI-32765 is highly active and well tolerated in patients (pts) with relapsed/refractory B cell malignancies: final results from a phase I study. Ann Oncol. 2011;22(Sppl 4):Abstract 153.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous