Oral hygiene in intensive care unit patients with photodynamic therapy: study protocol for randomised controlled trial
- PMID: 28830529
- PMCID: PMC5568342
- DOI: 10.1186/s13063-017-2133-y
Oral hygiene in intensive care unit patients with photodynamic therapy: study protocol for randomised controlled trial
Abstract
Background: In intensive care units (ICUs), nosocomial infections are prevalent conditions and they have been related to high mortality indexes. Some studies have suggested that inefficient oral hygiene and ventilator-associated pneumonia (VAP) are related. Nowadays, in the Brazilian public health system there is no well-defined protocol for oral hygiene in an ICU. Due to the drawbacks of the use of antibiotics, photodynamic therapy (PDT) has emerged as an interesting technique in order to reduce antimicrobial-resistant pathogens. Methylene blue (MB) is the most common chemical agent for PDT in Brazil. However, new formulations for improved effectiveness are still lacking. The objective of this study is to evaluate the use of an MB mouthwash as an effective oral-hygiene procedure in an ICU and to show that oral hygiene using PDT with MB mouthwash may reduce VAP frequency to rates similar to, or higher than, chlorhexidine.
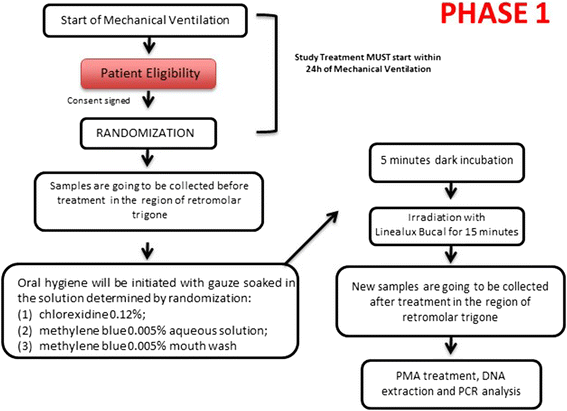
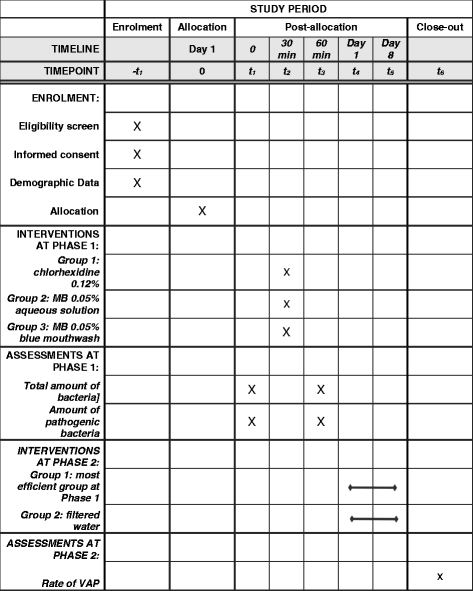
Methods: Phase 1 will evaluate the most effective cleaning procedure, while phase 2 will correlate oral hygiene to VAP incidence. At the start of phase 1, the ICU patients will be randomly allocated into three different groups (10 patients/group): the efficacy of chlorhexidine, classical MB-PDT, and mouthwash MB-PDT will all be measured for the quantification of viable bacteria, both pre- and post-treatment, by a Reverse Transcription Polymerase Chain Reaction (RT-PCR). In phase 2, the most effective procedure found in phase 1 and a mechanical cleaning with filtered water will be carried out daily, once a day, over 5 days, with a total of 52 ICU patients randomly allocated into the two groups. The clinical records will be evaluated in order to find any pneumonic diagnoses.
Discussion: Since a variety of bacterial species are related to VAP, a universal primer for bacteria will be used in order to quantify the total bacteria count in the participants' samples. In order to quantify only the living bacteria before DNA extraction, the samples will be treated with propidium monoazide. This will infiltrate the dead bacteria and will intercalate the DNA bases, avoiding their DNA amplification. This will be the first trial to evaluate MB-PDT in a mouthwash formula that can increase the effectiveness due to the control of MB aggregation. The results of this study will be able to generate an easy and low-cost protocol to be used in an ICU for the Brazilian public health system.
Trial registration: This protocol was approved by the Research Ethics Committee of the Conjunto Hospitalar do Mandaqui (1.317.834, CAAE: 49273515.9.3001.5551) and it was registered in Registro Brasileiro de Ensaios Clínicos (ReBEC number: RBR-94bvrc;). First received: 12 July 2015; 1st version 6 June 2016. Data will be published in a peer-reviewed journal.
Keywords: Dentistry; Intensive care units; Methylene blue mouthwash; Nosocomial infections; Photodynamic therapy.
Conflict of interest statement
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- de Silva FWG, Paula E, Queiroz AMD, Ito IY. Apical periodontitis: systemic repercussions? Odontol Clín Cient. 2010;9:299–302.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous