Membrane-anchored CCL20 augments HIV Env-specific mucosal immune responses
- PMID: 28830557
- PMCID: PMC5568278
- DOI: 10.1186/s12985-017-0831-4
Membrane-anchored CCL20 augments HIV Env-specific mucosal immune responses
Abstract
Background: Induction of broad immune responses at mucosal site remains a primary goal for most vaccines against mucosal pathogens. Abundance of evidence indicates that the co-delivery of mucosal adjuvants, including cytokines, is necessary to induce effective mucosal immunity. In the present study, we set out to evaluate the role of a chemokine, CCL20, as an effective mucosal adjuvant for HIV vaccine.
Methods: To evaluate the role of CCL20 as a potent adjuvant for HIV vaccine, we examined its effects on antigen-specific antibody responses, level of antibody-secreting cells, cytokine production and intestinal homing of plasma cells in vaccine immunized mice.
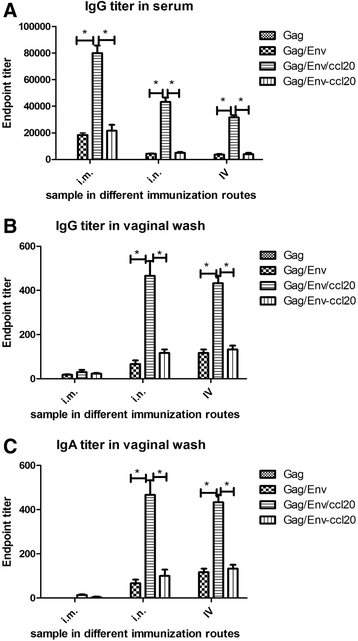
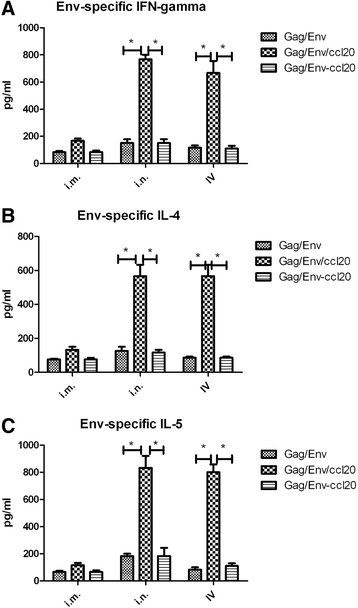
Results: CCL20-incorporated VLP administered by mucosal route (intranasal (n = 10, p = 0.0085) or intravaginal (n = 10, p = 0.0091)) showed much higher potency in inducing Env-specific IgA antibody response than those administered by intramuscular route (n = 10). For intranasal administration, the HIV Env-specific IFN-γ(751 pg/ml), IL-4 (566 pg/ml), IL-5 (811 pg/ml) production and IgA-secreting plasma cells (62 IgA-secreting plasma cells/106 cells) in mucosal lamina propria were significantly augmented in CCL20-incorporated VLP immunized mice as compared to those immunized with Env only VLPs (p = 0.0332, 0.0398, 0.033, 0.0302 for IFN-γ, IL-4, IL-5, and IgA-secreting plasma cells, respectively). Further, anti-CCL20 mAb partially suppressed homing of Env-specific IgA ASCs into small intestine in mice immunized with CCL20-incorporated VLP by intranasal (62 decreased to 16 IgA- secreting plasma cells/106 cells, p = 0.0341) or intravaginal (52 decreased to 13 IgA- secreting plasma cells/106 cells, p = 0.0332) routes.
Conclusion: Our data indicated that the VLP-incorporated CCL20 can enhance HIV Env-specific immune responses in mice, especially those occurring in the mucosal sites. We also found that i.m. prime followed by mucosal boost is critical and required for CCL20 to exert its full function as an effective mucosal adjuvant. Therefore, co-incorporation of CCL20 into Env VLPs when combined with mucosal administration could represent a novel and promising HIV vaccine candidate.
Keywords: Antibody-secreting cells (ASCs); CCL20; Chemokine; HIV vaccine; Mucosal immune responses; Virus-like particles (VLPs).
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Jiaxing University. The committee’s reference number was No.JUMC2016–001.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
CCL28 induces mucosal homing of HIV-1-specific IgA-secreting plasma cells in mice immunized with HIV-1 virus-like particles.PLoS One. 2011;6(10):e26979. doi: 10.1371/journal.pone.0026979. Epub 2011 Oct 31. PLoS One. 2011. PMID: 22066023 Free PMC article.
-
CTA1-DD adjuvant promotes strong immunity against human immunodeficiency virus type 1 envelope glycoproteins following mucosal immunization.J Gen Virol. 2008 Dec;89(Pt 12):2954-2964. doi: 10.1099/vir.0.2008/005470-0. J Gen Virol. 2008. PMID: 19008380
-
Intranasal administration of HIV-DNA vaccine formulated with a polymer, carboxymethylcellulose, augments mucosal antibody production and cell-mediated immune response.Clin Immunol Immunopathol. 1998 Aug;88(2):205-10. doi: 10.1006/clin.1998.4566. Clin Immunol Immunopathol. 1998. PMID: 9714699
-
The role of Th1 and Th2 cells for mucosal IgA responses.Ann N Y Acad Sci. 1996 Feb 13;778:64-71. doi: 10.1111/j.1749-6632.1996.tb21115.x. Ann N Y Acad Sci. 1996. PMID: 8611017 Review.
-
Interleukin 12 and innate molecules for enhanced mucosal immunity.Immunol Res. 1999;20(3):207-17. doi: 10.1007/BF02790404. Immunol Res. 1999. PMID: 10741861 Review.
Cited by
-
Chemokine-Adjuvanted Plasmid DNA Induces Homing of Antigen-Specific and Non-Antigen-Specific B and T Cells to the Intestinal and Genital Mucosae.J Immunol. 2020 Feb 15;204(4):903-913. doi: 10.4049/jimmunol.1901184. Epub 2020 Jan 8. J Immunol. 2020. PMID: 31915263 Free PMC article.
-
Oral Immunization with Attenuated Salmonella Choleraesuis Expressing the P42 and P97 Antigens Protects Mice against Mycoplasma hyopneumoniae Challenge.Microbiol Spectr. 2022 Dec 21;10(6):e0236122. doi: 10.1128/spectrum.02361-22. Epub 2022 Nov 15. Microbiol Spectr. 2022. PMID: 36377878 Free PMC article.
-
Parenterally Administered Porcine Epidemic Diarrhea Virus-Like Particle-Based Vaccine Formulated with CCL25/28 Chemokines Induces Systemic and Mucosal Immune Protectivity in Pigs.Viruses. 2020 Oct 2;12(10):1122. doi: 10.3390/v12101122. Viruses. 2020. PMID: 33023277 Free PMC article.
References
-
- Schulte R, Suh YS, Sauermann U, Ochieng W, Sopper S, Kim KS, Ahn SS, Park KS, Stolte-Leeb N, Hunsmann G, et al. Mucosal prior to systemic application of recombinant adenovirus boosting is more immunogenic than systemic application twice but confers similar protection against SIV-challenge in DNA vaccine-primed macaques. Virology. 2009;383:300–309. doi: 10.1016/j.virol.2008.10.012. - DOI - PubMed
-
- Matano T, Kobayashi M, Igarashi H, Takeda A, Nakamura H, Kano M, Sugimoto C, Mori K, Iida A, Hirata T, et al. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J Exp Med. 2004;199:1709–1718. doi: 10.1084/jem.20040432. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

