Contribution of amygdala CRF neurons to chronic pain
- PMID: 28830762
- PMCID: PMC5658242
- DOI: 10.1016/j.expneurol.2017.08.010
Contribution of amygdala CRF neurons to chronic pain
Abstract
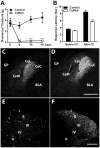
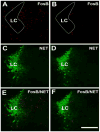
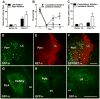
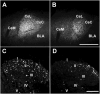
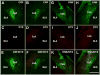
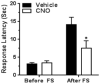
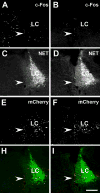
We investigated the role of amygdala corticotropin-releasing factor (CRF) neurons in the perturbations of descending pain inhibition caused by neuropathic pain. Forced swim increased the tail-flick response latency in uninjured mice, a phenomenon known as stress-induced analgesia (SIA) but did not change the tail-flick response latency in mice with neuropathic pain caused by sciatic nerve constriction. Neuropathic pain also increased the expression of CRF in the central amygdala (CeAmy) and ΔFosB in the dorsal horn of the spinal cord. Next, we injected the CeAmy of CRF-cre mice with cre activated AAV-DREADD (Designer Receptors Exclusively Activated by Designer Drugs) vectors. Activation of CRF neurons by DREADD/Gq did not affect the impaired SIA but inhibition of CRF neurons by DREADD/Gi restored SIA and decreased allodynia in mice with neuropathic pain. The possible downstream circuitry involved in the regulation of SIA was investigated by combined injections of retrograde cre-virus (CAV2-cre) into the locus ceruleus (LC) and cre activated AAV-diphtheria toxin (AAV-FLEX-DTX) virus into the CeAmy. The viral injections were followed by a sciatic nerve constriction ipsilateral or contralateral to the injections. Ablation of amygdala projections to the LC on the side of injury but not on the opposite side, completely restored SIA, decreased allodynia and decreased ΔFosB expression in the spinal cord in mice with neuropathic pain. The possible lateralization of SIA impairment to the side of injury was confirmed by an experiment in which unilateral inhibition of the LC decreased SIA even in uninjured mice. The current view in the field of pain research attributes the process of pain chronification to abnormal functioning of descending pain inhibition. Our results demonstrate that the continuous activity of CRF neurons brought about by persistent pain leads to impaired SIA, which is a symptom of dysregulation of descending pain inhibition. Therefore, an over-activation of amygdala CRF neurons is very likely an important contributing factor for pain chronification.
Keywords: Central amygdala; Chronic pain; Corticotropin-releasing factor; Descending pain inhibition; Neuropathic pain; Norepinephrine; Stress-induced analgesia.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
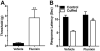

References
-
- Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–625. - PubMed
-
- Mayer DJ, Price DD. Central nervous system mechanisms of analgesia. Pain. 1976;2:379–404. - PubMed
-
- Liebeskind JC, Mayer DJ. Somatosensory evoked responses in the mesencephalic central gray matter of the rat. Brain Res. 1971;27:133–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

