Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia
- PMID: 28830889
- PMCID: PMC5649080
- DOI: 10.1182/blood-2017-04-780155
Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia
Abstract
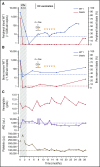
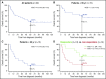
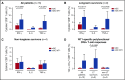
Relapse is a major problem in acute myeloid leukemia (AML) and adversely affects survival. In this phase 2 study, we investigated the effect of vaccination with dendritic cells (DCs) electroporated with Wilms' tumor 1 (WT1) messenger RNA (mRNA) as postremission treatment in 30 patients with AML at very high risk of relapse. There was a demonstrable antileukemic response in 13 patients. Nine patients achieved molecular remission as demonstrated by normalization of WT1 transcript levels, 5 of which were sustained after a median follow-up of 109.4 months. Disease stabilization was achieved in 4 other patients. Five-year overall survival (OS) was higher in responders than in nonresponders (53.8% vs 25.0%; P = .01). In patients receiving DCs in first complete remission (CR1), there was a vaccine-induced relapse reduction rate of 25%, and 5-year relapse-free survival was higher in responders than in nonresponders (50% vs 7.7%; P < .0001). In patients age ≤65 and >65 years who received DCs in CR1, 5-year OS was 69.2% and 30.8% respectively, as compared with 51.7% and 18% in the Swedish Acute Leukemia Registry. Long-term clinical response was correlated with increased circulating frequencies of polyepitope WT1-specific CD8+ T cells. Long-term OS was correlated with interferon-γ+ and tumor necrosis factor-α+ WT1-specific responses in delayed-type hypersensitivity-infiltrating CD8+ T lymphocytes. In conclusion, vaccination of patients with AML with WT1 mRNA-electroporated DCs can be an effective strategy to prevent or delay relapse after standard chemotherapy, translating into improved OS rates, which are correlated with the induction of WT1-specific CD8+ T-cell response. This trial was registered at www.clinicaltrials.gov as #NCT00965224.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: V.F.V.T. and Z.N.B. are coinventors of a patent covering the messenger RNA electroporation technique (WO/2003/000907; improved transfection of eukaryotic cells with linear polynucleotides by electroporation). Z.N.B. is a member of the Scientific Advisory Board of ExoCyte Therapeutics. The remaining authors declare no competing financial interests.
Figures

References
-
- Howlader N, Noone AM, Krapcho M, et al. . SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2012/browse_csr.php?sectionSEL=13&pageSE.... Accessed 31 December 2016.
-
- Dinmohamed AG, Visser O, van Norden Y, et al. . Treatment, trial participation and survival in adult acute myeloid leukemia: a population-based study in the Netherlands, 1989-2012. Leukemia. 2016;30(1):24-31. - PubMed
-
- Anguille S, Lion E, Smits E, Berneman ZN, van Tendeloo VF. Dendritic cell vaccine therapy for acute myeloid leukemia: questions and answers. Hum Vaccin. 2011;7(5):579-584. - PubMed
-
- van Besien K. Allogeneic transplantation for AML and MDS: GVL versus GVHD and disease recurrence. Hematology Am Soc Hematol Educ Program. 2013;2013:56-62. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

