The molecular chaperones DNAJB6 and Hsp70 cooperate to suppress α-synuclein aggregation
- PMID: 28831037
- PMCID: PMC5567236
- DOI: 10.1038/s41598-017-08324-z
The molecular chaperones DNAJB6 and Hsp70 cooperate to suppress α-synuclein aggregation
Erratum in
-
Author Correction: The molecular chaperones DNAJB6 and Hsp70 cooperate to suppress α-synuclein aggregation.Sci Rep. 2018 Aug 2;8(1):11848. doi: 10.1038/s41598-018-29893-7. Sci Rep. 2018. PMID: 30068912 Free PMC article.
Abstract
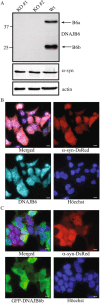
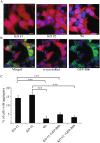
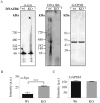
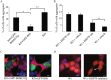
A major hallmark of Parkinson's disease (PD) is the presence of Lewy bodies (LBs) in certain neuronal tissues. LBs are protein-rich inclusions, in which α-synuclein (α-syn) is the most abundant protein. Since these inclusions are not present in healthy individuals, despite the high concentration of α-syn in neurons, it is important to investigate whether natural control mechanisms are present to efficiently suppress α-syn aggregation. Here, we demonstrate that a CRISPR/Cas9-mediated knockout (KO) of a DnaJ protein, DNAJB6, in HEK293T cells expressing α-syn, causes a massive increase in α-syn aggregation. Upon DNAJB6 re-introduction into these DNAJB6-KO HEK293T-α-syn cells, aggregation is reduced to the level of the parental cells. We then show that the suppression of α-syn aggregation is dependent on the J-domain of DNAJB6, as the catalytically inactive protein, which carries the H31Q mutation, does not suppress aggregation, when re-introduced into DNAJB6-KO cells. We further demonstrate, that the suppression of α-syn aggregation is dependent on the molecular chaperone Hsp70, which is consistent with the well-known function of J-domains of transferring unfolded and misfolded proteins to Hsp70. These data identify a natural control strategy to suppress α-syn aggregation and suggest potential therapeutic approaches to prevent or treat PD and related disorders.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

