Nm23-H1-stabilized hnRNPA2/B1 promotes internal ribosomal entry site (IRES)-mediated translation of Sp1 in the lung cancer progression
- PMID: 28831131
- PMCID: PMC5567229
- DOI: 10.1038/s41598-017-09558-7
Nm23-H1-stabilized hnRNPA2/B1 promotes internal ribosomal entry site (IRES)-mediated translation of Sp1 in the lung cancer progression
Abstract
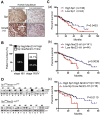
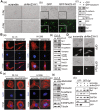
Our recent studies have indicated that specificity protein-1 (Sp1) accumulates substantially in the early stage of lung cancer but is partially decreased in the late stages, which is an important factor in the progression of the cancer. In this study, we found that Nm23-H1 and hnRNPA2/B1 could be recruited to the 5'UTR of Sp1 mRNA. In investigating the clinical relevance of Nm23-H1/Sp1 levels, we found a positive correlation between lung cancer patients with poor prognosis and low levels of Sp1 and Nm23-H1, suggesting an association between Nm23-H1/Sp1 levels and survival rate. Knockdown of Nm23-H1 inhibits lung cancer growth but increases lung cancer cell malignancy, which could be rescued by overexpression of Sp1, indicating that Nm23-H1-induced Sp1 expression is critical for lung cancer progression. We also found that Nm23-H1 increases the protein stability of hnRNPA2/B1and is thereby co-recruited to the 5'UTR of Sp1 mRNA to regulate cap-independent translational activity. Since the Sp1 level is tightly regulated during lung cancer progression, understanding the molecular mechanisms underlying the regulation by Nm23-H1/hnRNPA2B1 of Sp1 expression in the various stages of lung cancer will be beneficial for lung cancer therapy in the future.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Nm23-H1 inhibits lung cancer bone-specific metastasis by upregulating miR-660-5p targeted SMARCA5.Thorac Cancer. 2020 Mar;11(3):640-650. doi: 10.1111/1759-7714.13308. Epub 2020 Feb 5. Thorac Cancer. 2020. PMID: 32022430 Free PMC article.
-
[Molecular mechanism of reversing metastatic phenotype in human high-metastatic large cell lung cancer cell line L9981 by nm23-H1].Ai Zheng. 2005 Mar;24(3):278-84. Ai Zheng. 2005. PMID: 15757527 Chinese.
-
Co-downregulation of PTEN, KAI-1, and nm23-H1 tumor/metastasis suppressor proteins in non-small cell lung cancer.Ann Diagn Pathol. 2004 Feb;8(1):6-16. doi: 10.1016/j.anndiagpath.2003.11.002. Ann Diagn Pathol. 2004. PMID: 15129904
-
The multiple regulation of metastasis suppressor NM23-H1 in cancer.Life Sci. 2021 Mar 1;268:118995. doi: 10.1016/j.lfs.2020.118995. Epub 2021 Jan 6. Life Sci. 2021. PMID: 33421524 Review.
-
Targeting tumor metastasis by regulating Nm23 gene expression.Asian Pac J Cancer Prev. 2012;13(8):3539-48. doi: 10.7314/apjcp.2012.13.8.3539. Asian Pac J Cancer Prev. 2012. PMID: 23098432 Review.
Cited by
-
Construction of a Comprehensive Diagnostic Scoring Model for Prostate Cancer Based on a Novel Six-Gene Panel.Front Genet. 2022 Apr 26;13:831162. doi: 10.3389/fgene.2022.831162. eCollection 2022. Front Genet. 2022. PMID: 35559023 Free PMC article.
-
CRNDE mediated hnRNPA2B1 stability facilitates nuclear export and translation of KRAS in colorectal cancer.Cell Death Dis. 2023 Sep 16;14(9):611. doi: 10.1038/s41419-023-06137-9. Cell Death Dis. 2023. PMID: 37716979 Free PMC article.
-
Targeting HNRNPA2B1 to overcome chemotherapy resistance in gastric cancer stem cells: Mechanisms and therapeutic potential.J Biol Chem. 2025 Apr;301(4):108234. doi: 10.1016/j.jbc.2025.108234. Epub 2025 Jan 25. J Biol Chem. 2025. PMID: 39870196 Free PMC article.
-
Heterogeneous nuclear ribonucleoprotein A/B: an emerging group of cancer biomarkers and therapeutic targets.Cell Death Discov. 2022 Jul 25;8(1):337. doi: 10.1038/s41420-022-01129-8. Cell Death Discov. 2022. PMID: 35879279 Free PMC article. Review.
-
Estradiol-mediated inhibition of Sp1 decreases miR-3194-5p expression to enhance CD44 expression during lung cancer progression.J Biomed Sci. 2022 Jan 17;29(1):3. doi: 10.1186/s12929-022-00787-1. J Biomed Sci. 2022. PMID: 35034634 Free PMC article.
References
-
- Opitz OG, Rustgi AK. Interaction between Sp1 and cell cycle regulatory proteins is important in transactivation of a differentiation-related gene. Cancer Res. 2000;60:2825–2830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

