IL-10-Producing B Cells Suppress Effector T Cells Activation and Promote Regulatory T Cells in Crystalline Silica-Induced Inflammatory Response In Vitro
- PMID: 28831210
- PMCID: PMC5558645
- DOI: 10.1155/2017/8415094
IL-10-Producing B Cells Suppress Effector T Cells Activation and Promote Regulatory T Cells in Crystalline Silica-Induced Inflammatory Response In Vitro
Abstract
Long-term exposure to crystalline silica leads to silicosis, which is characterized by persistent lung inflammation and lung fibrosis. Multiple immune cells have been demonstrated to participate in crystalline silica-induced immune responses. Our previous study indicated that B10 could control lung inflammation through modulating the Th balance in experimental silicosis in mice. However, the regulatory mechanism of B10 on CD4+ T cells is still unclear. MACS-sorted CD19+ B cells from the three different groups were cultured with CD4+ T cells either with or without transwell insert plates to evaluate the effects of B10 on CD4+ T cells, including Teff and Treg. B10 was eliminated by anti-CD22 application in vivo. Flow cytometry was used to test the frequencies of CD4+ T cells, and the expressions of the related cytokines were detected by real-time PCR and CBA. Insufficient B10 elevated the levels of proinflammatory cytokines and promoted Th responses in a way independent upon cell-cell contact in the Teff and B cell coculture system. B10 could both increase Treg activity and enhance conversion of Teff into Treg. Our findings demonstrated that B10 could affect Th responses by the release of IL-10, enhancing Treg functions and converting Teff into Treg.
Figures
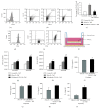
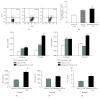
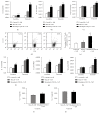
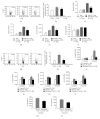
Similar articles
-
Role of IL-10-producing regulatory B cells in modulating T-helper cell immune responses during silica-induced lung inflammation and fibrosis.Sci Rep. 2016 Jun 29;6:28911. doi: 10.1038/srep28911. Sci Rep. 2016. PMID: 27354007 Free PMC article.
-
CD4+Foxp3+ regulatory T cells converted by rapamycin from peripheral CD4+CD25(-) naive T cells display more potent regulatory ability in vitro.Chin Med J (Engl). 2010 Apr 5;123(7):942-8. Chin Med J (Engl). 2010. PMID: 20497692
-
The IL-10-producing regulatory B cells (B10 cells) and regulatory T cell subsets in neuromyelitis optica spectrum disorder.Neurol Sci. 2018 Mar;39(3):543-549. doi: 10.1007/s10072-018-3248-y. Epub 2018 Jan 19. Neurol Sci. 2018. PMID: 29349658
-
Regulatory B and T cell responses in patients with autoimmune thyroid disease and healthy controls.Dan Med J. 2016 Feb;63(2):B5177. Dan Med J. 2016. PMID: 26836805 Review.
-
IL-10 producing regulatory B cells in mice and humans: state of the art.Curr Mol Med. 2012 Jun;12(5):519-27. doi: 10.2174/156652412800620057. Curr Mol Med. 2012. PMID: 22292445 Review.
Cited by
-
Altered Regulatory B Cell Subsets in Children with Type 1 Diabetes Mellitus.J Immunol Res. 2020 Jul 21;2020:8935694. doi: 10.1155/2020/8935694. eCollection 2020. J Immunol Res. 2020. PMID: 32775471 Free PMC article.
-
Comparative Transcriptome Analyses Reveal a Transcriptional Landscape of Human Silicosis Lungs and Provide Potential Strategies for Silicosis Treatment.Front Genet. 2021 Jun 3;12:652901. doi: 10.3389/fgene.2021.652901. eCollection 2021. Front Genet. 2021. PMID: 34149803 Free PMC article.
-
Interpreting Immunoregulation in Lung Fibrosis: A New Branch of the Immune Model.Front Immunol. 2021 Aug 20;12:690375. doi: 10.3389/fimmu.2021.690375. eCollection 2021. Front Immunol. 2021. PMID: 34489937 Free PMC article. Review.
-
Mouse innate-like B-1 lymphocytes promote inhaled particle-induced in vitro granuloma formation and inflammation in conjunction with macrophages.Arch Toxicol. 2022 Feb;96(2):585-599. doi: 10.1007/s00204-021-03200-2. Epub 2021 Dec 21. Arch Toxicol. 2022. PMID: 34935064 Free PMC article.
-
TLR9 Signaling Is Required for the Porphyromonas gingivalis-Induced Activation of IL-10-Expressing B Cells.Int J Mol Sci. 2023 Apr 3;24(7):6693. doi: 10.3390/ijms24076693. Int J Mol Sci. 2023. PMID: 37047666 Free PMC article.
References
-
- Lee J. W., Myong J. P., Choi Y. J., Lee S., Jo B. S., Koo J. W. Diagnosis of perinuclear anti-neutrophil cytoplasmic antibody-associated microscopic polyangiitis in silicotics: case report. Annals of Occupational and Environmental Medicine. 2016;28:p. 21. doi: 10.1186/s40557-016-0108-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

