Ameliorative Effect of Daidzein on Cisplatin-Induced Nephrotoxicity in Mice via Modulation of Inflammation, Oxidative Stress, and Cell Death
- PMID: 28831294
- PMCID: PMC5558675
- DOI: 10.1155/2017/3140680
Ameliorative Effect of Daidzein on Cisplatin-Induced Nephrotoxicity in Mice via Modulation of Inflammation, Oxidative Stress, and Cell Death
Abstract
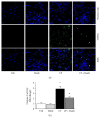
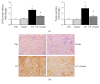
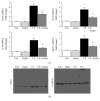
Oxidative stress and inflammation are part and parcel of cisplatin-induced nephrotoxicity. The purpose of this work is to study the role of soy isoflavone constituent, daidzein, in cisplatin-induced renal damage. Cisplatin-induced nephrotoxicity was evident by the histological damage in proximal tubular cells and by the increase in serum neutrophil gelatinase-associated lipocalin (NGAL), blood urea nitrogen (BUN), creatinine, and urinary kidney injury molecule-1 (KIM-1). Cisplatin-induced cell death was shown by TUNEL staining and caspase-3/7 activity. Daidzin treatment reduced all kidney injury markers (NGAL, BUN, creatinine, and KIM-1) and attenuated cell death (apoptotic markers). In cisplatin-induced kidney injury, renal oxidative/nitrative stress was manifested by the increase in lipid peroxidation and protein nitration. Cisplatin induced the reactive oxygen species-generating enzyme NOX-2 and impaired antioxidant defense enzyme activities such as glutathione peroxidase (GPX) and superoxide dismutase (SOD) activities. Cisplatin-induced oxidative/nitrative stress was attenuated by daidzein treatment. Cisplatin induced CD11b-positive macrophages in kidneys and daidzein attenuated CD11b-positive cells. Daidzein attenuated cisplatin-induced inflammatory cytokines tumor necrosis factor α (TNFα), interleukin 10 (IL-10), interleukin 18 (IL-18), and monocyte chemoattractant protein-1 (MCP-1). Daidzein attenuated cell death in vitro. Our data suggested that daidzein attenuated cisplatin-induced kidney injury through the downregulation of oxidative/nitrative stress, immune cells, inflammatory cytokines, and apoptotic cell death, thus improving kidney regeneration.
Figures





Similar articles
-
Protective effect of urolithin a on cisplatin-induced nephrotoxicity in mice via modulation of inflammation and oxidative stress.Food Chem Toxicol. 2019 Jul;129:108-114. doi: 10.1016/j.fct.2019.04.031. Epub 2019 Apr 20. Food Chem Toxicol. 2019. PMID: 31014901
-
Mitochondrial modulation by Epigallocatechin 3-Gallate ameliorates cisplatin induced renal injury through decreasing oxidative/nitrative stress, inflammation and NF-kB in mice.PLoS One. 2015 Apr 15;10(4):e0124775. doi: 10.1371/journal.pone.0124775. eCollection 2015. PLoS One. 2015. PMID: 25875356 Free PMC article.
-
Amelioration of Cisplatin-induced Renal Inflammation by Recombinant Human Golimumab in Mice.Curr Pharm Biotechnol. 2022;23(7):970-977. doi: 10.2174/1389201022666210810141139. Curr Pharm Biotechnol. 2022. PMID: 35135447
-
Mitochondrial dysregulation and protection in cisplatin nephrotoxicity.Arch Toxicol. 2014 Jun;88(6):1249-56. doi: 10.1007/s00204-014-1239-1. Epub 2014 May 24. Arch Toxicol. 2014. PMID: 24859930 Free PMC article. Review.
-
Daidzin and its de-glycosylated constituent daidzein as a potential therapeutic for cardiovascular diseases: A review from bench to bed.Phytother Res. 2024 Aug;38(8):3973-3985. doi: 10.1002/ptr.8261. Epub 2024 Jun 7. Phytother Res. 2024. PMID: 38847155 Review.
Cited by
-
Effects of Soy Bread on Cardiovascular Risk Factor, Inflammation and Oxidative Stress in Women With Active Rheumatoid Arthritis: A Randomized Double-Blind Controlled Trial.Clin Nutr Res. 2024 Jan 23;13(1):22-32. doi: 10.7762/cnr.2024.13.1.22. eCollection 2024 Jan. Clin Nutr Res. 2024. PMID: 38362131 Free PMC article.
-
Protective Effects of Orexin A in a Murine Model of Cisplatin-Induced Acute Kidney Injury.J Clin Med. 2022 Dec 3;11(23):7196. doi: 10.3390/jcm11237196. J Clin Med. 2022. PMID: 36498769 Free PMC article.
-
Effect of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on cisplatin-induced nephrotoxicity in mice.Naunyn Schmiedebergs Arch Pharmacol. 2019 Jan;392(1):45-53. doi: 10.1007/s00210-018-1564-7. Epub 2018 Sep 11. Naunyn Schmiedebergs Arch Pharmacol. 2019. PMID: 30206656
-
Unveiling the Pharmacological and Nanotechnological Facets of Daidzein: Present State-of-the-Art and Future Perspectives.Molecules. 2023 Feb 13;28(4):1765. doi: 10.3390/molecules28041765. Molecules. 2023. PMID: 36838751 Free PMC article. Review.
-
A review on the traditional uses, nutritive importance, pharmacognostic features, phytochemicals, and pharmacology of Momordica cymbalaria Hook F.PeerJ. 2024 Feb 27;12:e16928. doi: 10.7717/peerj.16928. eCollection 2024. PeerJ. 2024. PMID: 38436002 Free PMC article. Review.
References
-
- Santos N. A., Bezerra C. S., Martins N. M., Curti C., Bianchi M. L., Santos A. C. Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemotherapy and Pharmacology. 2008;61:145–155. doi: 10.1007/s00280-007-0459-y. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

