Vismodegib for Locally Advanced Periocular and Orbital Basal Cell Carcinoma: A Review of 15 Consecutive Cases
- PMID: 28831360
- PMCID: PMC5548583
- DOI: 10.1097/GOX.0000000000001424
Vismodegib for Locally Advanced Periocular and Orbital Basal Cell Carcinoma: A Review of 15 Consecutive Cases
Abstract
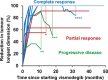
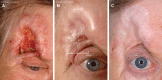
Basal cell carcinoma (BCC) is the most common periocular skin cancer and can lead to significant morbidity. We assess the effectiveness of vismodegib, a first-in-class Hedgehog signaling pathway inhibitor, in the management of periocular and orbital BCCs based on clinical response, tolerability, and orbital content preservation. All patients with periocular or orbital BCCs who met criteria for vismodegib treatment were recruited prospectively between May 2012 and 2014 from 2 hospitals. Patients received oral vismodegib (150 mg daily) until disease progression, unacceptable toxicity, or withdrawal. All patients were followed up monthly. Patient demographics, tumor size, treatment duration including dosing regimen, adverse events, response rate, duration of response, progression-free survival, and disease-free survival were analyzed. All 15 patients had biopsy-proven BCCs with no metastatic disease at presentation. The mean age was 74 years and 10 patients (67%) had orbital involvement. The mean lesion longest dimension was 51 mm and 7 cases (47%) represented recurrence following previous surgery and/or radiotherapy. The mean treatment duration was 13 months and mean follow-up duration 36 months. Ten patients (67%) had a complete response, 3 (20%) had a partial response, and 2 had progressive disease following an initial partial response (13%). The partial response of 55% in 1 patient allowed subsequent surgical resection with clear margins. Vismodegib is effective for treating periocular and orbital BCCs with orbital salvage of patients who otherwise would have required exenteration. There is a neoadjuvant role for vismodegib but further studies are required.
Conflict of interest statement
Disclosure: Drs. Fife, Lear, and Durrani have received honoraria from Roche for speaker and advisory board meetings. Dr. Fife has also received honoraria from Pfizer, Novartis, and Bristol-Myers Squibb for speaker and advisory board meetings; support of trials at Cambridge Cancer Centre from Roche (STEVIE trial) and other companies (renal cancer). Dr. Wong and Dr. Price have no conflicts of interest or financial disclosures to declare. The Article Processing Charge was paid for by the authors.
Figures
References
-
- Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375:673–685.. - PubMed
-
- Bath-Hextall F, Leonardi-Bee J, Smith C, et al. Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study. Int J Cancer. 2007;121:2105–2108.. - PubMed
-
- Cook BE, Jr, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County, Minnesota. Ophthalmology. 1999;106:746–750.. - PubMed
-
- Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma. Study of a series of 1039 consecutive neoplasms. J Am Acad Dermatol. 1990;23:1118–1126.. - PubMed
-
- Howard GR, Nerad JA, Carter KD, et al. Clinical characteristics associated with orbital invasion of cutaneous basal cell and squamous cell tumors of the eyelid. Am J Ophthalmol. 1992;113:123–133.. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
