Survey of Implementation of Antiemetic Prescription Standards in Indian Oncology Practices and Its Adherence to the American Society of Clinical Oncology Antiemetic Clinical Guideline
- PMID: 28831443
- PMCID: PMC5560456
- DOI: 10.1200/JGO.2016.006023
Survey of Implementation of Antiemetic Prescription Standards in Indian Oncology Practices and Its Adherence to the American Society of Clinical Oncology Antiemetic Clinical Guideline
Abstract
Purpose: Adherence to international antiemetic prophylaxis guidelines like those of ASCO can result in better control of chemotherapy-induced nausea and vomiting; however, the extent of implementation of such guidelines in India is unknown. Therefore, this survey was planned.
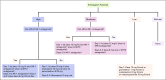
Methods: This study was an anonymized cross-sectional survey approved by the ethics committee. Survey items were generated from the clinical questions given in the ASCO guidelines. The survey was disseminated through personal contacts at an oncology conference and via e-mail to various community oncology centers across India. The B1, B2, and B3 domains included questions regarding the optimal antiemetic prophylaxis for high, moderate, and low-minimal emetogenic regimens.
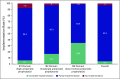
Results: Sixty-six (62.9%) of 105 responded and 65 centers (98.5%) were aware of the published guidelines. The partial, full, and no implementation scores were 92.5%, 4.5%, and 3.0%, respectively. Full implementation was better for the low-minimal emetogenic regimens (34.8%) than the highly emetogenic regimens (6.1%). The three most frequent reasons for hampered implementation of ASCO guidelines in routine chemotherapy practice cited by centers were a lack of sensitization (26 centers; 39.4%), lack of national guidelines (12 centers; 18.2%), and lack of administrative support (10 centers; 15.2%).
Conclusion: Awareness regarding ASCO antiemetic guidelines is satisfactory in Indian oncology practices; however, there is a need for sensitization of oncologists toward complete implementation of these guidelines in their clinical practice.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc. Vijay PatilNo relationship to discloseVanita NoronhaNo relationship to discloseAmit JoshiNo relationship to disclosePurvish ParikhNo relationship to discloseAtanu BhattacharjeeNo relationship to discloseSantam ChakrabortyNo relationship to discloseSunny JandyalNo relationship to discloseVamshi MudduNo relationship to discloseAnant RamaswamyNo relationship to discloseK. Govinda BabuNo relationship to discloseNilesh LokeshwarConsulting or Advisory Role: Bristol-Myers Squibb, Eisai, Dr. Reddy's Laboratories Travel, Accommodations, Expenses: Dr. Reddy's LaboratoriesSachin HingmireNo relationship to discloseNikhil GhadyalpatilNo relationship to discloseShripad BanavaliNo relationship to discloseKumar PrabhashNo relationship to disclose
Figures
References
-
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. - PubMed
-
- Pirri C, Bayliss E, Trotter J, et al. Nausea still the poor relation in antiemetic therapy? The impact on cancer patients’ quality of life and psychological adjustment of nausea, vomiting and appetite loss, individually and concurrently as part of a symptom cluster. Support Care Cancer. 2013;21:735–748. - PubMed
-
- Bloechl-Daum B, Deuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–4478. - PubMed
-
- Perwitasari DA, Atthobari J, Mustofa M, et al. Impact of chemotherapy-induced nausea and vomiting on quality of life in Indonesian patients with gynecologic cancer. Int J Gynecol Cancer. 2012;22:139–145. - PubMed
-
- Moore S, Tumeh J, Wojtanowski S, et al. Cost-effectiveness of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy. Value Health. 2007;10:23–31. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

