Structural Analysis of Unsaturated Glycosphingolipids Using Shotgun Ozone-Induced Dissociation Mass Spectrometry
- PMID: 28831744
- PMCID: PMC5647240
- DOI: 10.1007/s13361-017-1772-2
Structural Analysis of Unsaturated Glycosphingolipids Using Shotgun Ozone-Induced Dissociation Mass Spectrometry
Abstract
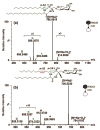
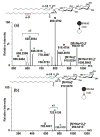
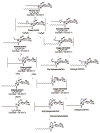
Glycosphingolipids are essential biomolecules widely distributed across biological kingdoms yet remain relatively underexplored owing to both compositional and structural complexity. While the glycan head group has been the subject of most studies, there is paucity of reports on the lipid moiety, particularly the location of unsaturation. In this paper, ozone-induced dissociation mass spectrometry (OzID-MS) implemented in a traveling wave-based quadrupole time-of-flight (Q-ToF) mass spectrometer was applied to study unsaturated glycosphingolipids using shotgun approach. Resulting high resolution mass spectra facilitated the unambiguous identification of diagnostic OzID product ions. Using [M+Na]+ adducts of authentic standards, we observed that the long chain base and fatty acyl unsaturation had distinct reactivity with ozone. The reactivity of unsaturation in the fatty acyl chain was about 8-fold higher than that in the long chain base, which enables their straightforward differentiation. Influence of the head group, fatty acyl hydroxylation, and length of fatty acyl chain on the oxidative cleavage of double bonds was also observed. Application of this technique to bovine brain galactocerebrosides revealed co-isolated isobaric and regioisomeric species, which otherwise would be incompletely identified using contemporary collision-induced dissociation (CID) alone. These results highlight the potential of OzID-MS in glycosphingolipids research, which not only provides complementary structural information to existing CID technique but also facilitates de novo structural determination of these complex biomolecules. Graphical Abstract ᅟ.
Keywords: Accurate mass measurement; Cerebrosides; Globosides; Glycosphingolipids; Ozone-induced dissociation.
Figures




References
-
- Varki A, Cummings R, Esko J. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2009.
-
- Boomkamp SD, Butters TD. In: Glycosphingolipid Disorders of the Brain. Quinn PJ, Wang X, editors. Springer; Netherlands, Dordrecht: 2008. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

