Longitudinal Assessment of the Effect of Atrasentan on Thoracic Bioimpedance in Diabetic Nephropathy: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 28831752
- PMCID: PMC5629141
- DOI: 10.1007/s40268-017-0201-0
Longitudinal Assessment of the Effect of Atrasentan on Thoracic Bioimpedance in Diabetic Nephropathy: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Background: Fluid retention is a common adverse event in patients who receive endothelin (ET) receptor antagonist therapy, including the highly selective ETA receptor antagonist, atrasentan.
Objective: We performed longitudinal assessments of thoracic bioimpedance in patients with type 2 diabetes mellitus and nephropathy to determine whether a decrease in bioimpedance accurately reflected fluid retention during treatment with atrasentan.
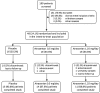
Study design: We conducted a randomized, double-blind, placebo-controlled study in 48 patients with type 2 diabetes mellitus and nephropathy who were receiving stable doses of renin angiotensin system inhibitors and diuretics.
Methods: Patients were randomized 1:1:1 to placebo, atrasentan 0.5 mg, or atrasentan 1.25 mg once daily for 8 weeks. Thoracic bioimpedance, vital signs, clinical exams, and serologies were taken at weeks 1, 2, 4, 6, and 8, with the exception of serum hemoglobin, which was not taken at week 1, and serum brain natriuretic peptide, which was only taken at baseline, week 4, and week 8.
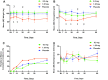
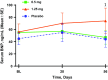
Results: Alterations in bioimpedance were more often present in those who received atrasentan than in those who received placebo, though overall differences were not statistically significant. Transient declines in thoracic bioimpedance during the first 2 weeks of atrasentan exposure occurred before or during peak increases in body weight and hemodilution (decreased serum hemoglobin).
Conclusions: We conclude that thoracic bioimpedance did not reflect changes in weight gain or edema with atrasentan treatment in this study. However, the sample size was small, and it may be of interest to explore the use of thoracic bioimpedance in a larger population to understand its potential clinical use in monitoring fluid retention in patients with chronic kidney disease who receive ET receptor antagonists.
Conflict of interest statement
Ethical standards
The study was conducted in accordance with the International Conference on Harmonisation guidelines, applicable regulations, and the principles of the Declaration of Helsinki. The study protocol was approved by an independent ethics committee or institutional review board.
Informed consent
All patients provided written informed consent.
Funding
AbbVie funded the study and was responsible for the study design, research, analysis, and data collection. The authors were responsible for interpretation of data, for writing, reviewing, and approving the manuscript and for determining the final content. No payments were made to the authors for writing this paper. Editorial support was provided by Richard M. Edwards, PhD, of Complete Publication Solutions, LLC (North Wales, PA, USA), a CHC Group company, and was funded by AbbVie.
Conflict of interest
DJW is a consultant for AbbVie and has been a member of the independent data monitoring boards for the SONAR (Study Of diabetic Nephropathy with AtRasentan) and RADAR (Reducing residual Albuminuria in subjects with Diabetes and nephropathy With AtRasentan) clinical trials. RB serves on the speakers’ bureau of AbbVie, Merck, Bristol-Myers Squibb, Novo Nordisk, Daiichi Sankyo, Takeda, Eli Lilly, and Sanofi. PP is a consultant for AbbVie. DdZ is a consultant for and receives honoraria (to employer) from AbbVie, Astellas, AstraZeneca, Chemocentryx, J&J, Hemocue, Novartis, Reata, Takeda, and Vitae. RC-R has consultancy agreements with Roche, AbbVie, Amgen, AstraZeneca, and Boehringer Ingelheim; in the last 2 years he has served on the speakers’ bureau of Amgen, Roche, and Sanofi, and received grant support from Fibrogen and Janssen. DK is a consultant for AbbVie. HJLH is a consultant for and receives honoraria (to employer) from AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, and Janssen. HM is a consultant for AbbVie and Teijin; receives speaker honoraria from Astellas, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo, Kyowa Hakko Kirin, MSD, Pfizer, Takeda, and Tanabe Mitsubishi; and receives grant support from Astellas, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo, Kowa, Kyowa Hakko Kirin, MSD, Novartis, Novo Nordisk, Ono, Otsuka, Pfizer, Taishyo-Toyama, Takeda, Teijin, and Tanabe Mitsubishi. VP is a consultant for AbbVie, Astellas, AstraZeneca, Boehringer, Janssen, Roche, Servier, Merck, and Vitae. His employer receives funding/contracts for clinical trials from AbbVie, Baxter, Fresenius, Novartis, Pfizer, Resmed, Roche, Janssen, and Servier. YP is a former employee of Abbott and owns AbbVie stocks. GR is a consultant for Alexion, Reata, Bayer, Novartis, and AbbVie. All compensations are paid to his institution for research and educational activities. SWT is a consultant for AbbVie and received honoraria for academic talks from Servier. He is an investigator on both contract and investigator-initiated research projects with AstraZeneca, Bristol-Myers Squibb, Mitsubishi, and Pfizer. RT serves on advisory boards for Boehringer Ingelheim, Amgen, Relypsa, ZS Pharma, Bayer, Quintiles, and AstraZeneca. H-HP is a consultant for AbbVie. BC, DA, JJB, and MH are all AbbVie employees and own AbbVie stock.
Figures
References
-
- Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117(23):3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

