Reflectance confocal microscopy-guided laser ablation of basal cell carcinomas: initial clinical experience
- PMID: 28831793
- PMCID: PMC5566590
- DOI: 10.1117/1.JBO.22.8.085005
Reflectance confocal microscopy-guided laser ablation of basal cell carcinomas: initial clinical experience
Abstract
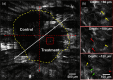
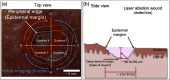
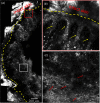
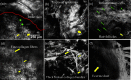
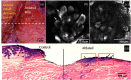
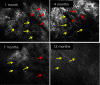
Laser ablation offers a procedure for precise, fast, and minimally invasive removal of superficial and early nodular basal cell carcinomas (BCCs). However, the lack of histopathological confirmation has been a limitation toward widespread use in the clinic. A reflectance confocal microscopy (RCM) imaging-guided approach offers cellular-level histopathology-like feedback directly on the patient, which may then guide and help improve the efficacy of the ablation procedure. Following an ex vivo benchtop study (reported in our earlier papers), we performed an initial study on 44 BCCs on 21 patients in vivo, using a pulsed erbium:ytterbium aluminum garnet laser and a contrast agent (aluminum chloride). In 10 lesions on six patients, the RCM imaging-guided detection of either presence of residual tumor or complete clearance was immediately confirmed with histopathology. Additionally, 34 BCCs on 15 patients were treated with RCM imaging-guided laser ablation, with immediate confirmation for clearance of tumor (no histopathology), followed by longer-term monitoring, currently in progress, with follow-up imaging (again, no histopathology) at 3, 6, and 18 months. Thus far, the imaging resolution appears to be sufficient and consistent for monitoring efficacy of ablation in the wound, both immediately postablation and subsequently during recovery. The efficacy results appear to be promising, with observed clearance in 19 cases of 22 cases with follow-ups ranging from 6 to 21 months. An additional 12 cases with 1 to 3 months of follow-ups has shown clearance of tumor but a longer follow-up time is required to establish conclusive results. Further instrumentation development will be necessary to cover larger areas with a more automatically controlled instrument for more uniform, faster, and deeper imaging of margins.
Keywords: basal cell carcinoma; confocal microscopy; image-guided therapy; laser ablation; skin cancer.
(2017) COPYRIGHT Society of Photo-Optical Instrumentation Engineers (SPIE).
Figures

Similar articles
-
Confocal microscopy to guide erbium:yttrium aluminum garnet laser ablation of basal cell carcinoma: an ex vivo feasibility study.J Biomed Opt. 2013 Sep;18(9):095001. doi: 10.1117/1.JBO.18.9.095001. J Biomed Opt. 2013. PMID: 24045654 Free PMC article.
-
Confocal imaging of carbon dioxide laser-ablated basal cell carcinomas: An ex-vivo study on the uptake of contrast agent and ablation parameters.Lasers Surg Med. 2016 Feb;48(2):133-9. doi: 10.1002/lsm.22415. Epub 2015 Sep 22. Lasers Surg Med. 2016. PMID: 26392001 Free PMC article.
-
Reflectance confocal microscopy-guided carbon dioxide laser ablation of low-risk basal cell carcinomas: A prospective study.J Am Acad Dermatol. 2019 Oct;81(4):984-988. doi: 10.1016/j.jaad.2019.06.014. Epub 2019 Jun 14. J Am Acad Dermatol. 2019. PMID: 31202871 Free PMC article.
-
Role of In Vivo Reflectance Confocal Microscopy in the Analysis of Melanocytic Lesions.Acta Dermatovenerol Croat. 2018 Apr;26(1):64-67. Acta Dermatovenerol Croat. 2018. PMID: 29782304 Review.
-
In Vivo and Ex Vivo Confocal Microscopy for Dermatologic and Mohs Surgeons.Dermatol Clin. 2016 Oct;34(4):497-504. doi: 10.1016/j.det.2016.05.012. Dermatol Clin. 2016. PMID: 27692455 Free PMC article. Review.
Cited by
-
Evaluation of a Combined Reflectance Confocal Microscopy-Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma.JAMA Dermatol. 2018 Oct 1;154(10):1175-1183. doi: 10.1001/jamadermatol.2018.2446. JAMA Dermatol. 2018. PMID: 30140851 Free PMC article.
-
A Prospective Double-Blinded Comparison of Reflectance Confocal Microscopy with Conventional Histopathology for In Vivo Assessment in Oral Cancer.Clin Cancer Res. 2024 Jun 3;30(11):2486-2496. doi: 10.1158/1078-0432.CCR-23-1361. Clin Cancer Res. 2024. PMID: 38526414 Free PMC article.
-
Skin Cancer Pathobiology at a Glance: A Focus on Imaging Techniques and Their Potential for Improved Diagnosis and Surveillance in Clinical Cohorts.Int J Mol Sci. 2023 Jan 5;24(2):1079. doi: 10.3390/ijms24021079. Int J Mol Sci. 2023. PMID: 36674595 Free PMC article. Review.
-
Feasibility of a Video-Mosaicking Approach to Extend the Field-of-View For Reflectance Confocal Microscopy in the Oral Cavity In Vivo.Lasers Surg Med. 2019 Jul;51(5):439-451. doi: 10.1002/lsm.23090. Epub 2019 May 8. Lasers Surg Med. 2019. PMID: 31067360 Free PMC article.
-
In vivo imaging characterization of basal cell carcinoma and cutaneous response to high-dose ionizing radiation therapy: A prospective study of reflectance confocal microscopy, dermoscopy, and ultrasonography.J Am Acad Dermatol. 2021 Jun;84(6):1575-1584. doi: 10.1016/j.jaad.2020.07.130. Epub 2020 Aug 20. J Am Acad Dermatol. 2021. PMID: 32827607 Free PMC article. Clinical Trial.
References
-
- Greenway H. T, et al. , “Mohs microscopic surgery and cutaneous oncology,” in Surgery of the Skin: Procedural Dermatology, Robinson J. K., et al., Eds., 3rd ed., Elsevier Saunders; (2015).
-
- Goldenberg G., Hamid O., “Nonsurgical treatment options for basal cell carcinoma—focus on advanced disease,” J. Drugs Dermatol. 12(12), 1369–1378 (2013). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

