Racial differences in calculated bioavailable vitamin D with vitamin D/calcium supplementation
- PMID: 28832406
- PMCID: PMC5656526
- DOI: 10.1097/QAD.0000000000001621
Racial differences in calculated bioavailable vitamin D with vitamin D/calcium supplementation
Abstract
Objectives: Some studies suggest that bioavailable 25-dihydroxyvitamin D [25-(OH)D] is more accurate than total 25-(OH)D as an assessment of vitamin D (VitD) status in black individuals. We hypothesized that increases in bioavailable 25-(OH)D would correlate better with improvement in bone outcomes among black HIV-infected adults.
Design: This is a secondary analysis of AIDS Clinical Trials Group A5280, a randomized, double-blind study of VitD3 and calcium supplementation in HIV-infected participants initiating antiretroviral therapy.
Methods: Effect of VitD/calcium on total and calculated bioavailable 25-(OH)D, parathyroid hormone, bone turnover markers, and bone mineral density in black and nonblack participants were evaluated at 48 weeks. Wilcoxon signed-rank tests and Wilcoxon rank sum tests assessed within and between-race differences.
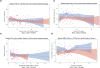
Results: Of 165 participants enrolled, 129 (40 black and 89 nonblack) had complete data. At baseline, black participants had lower total 25-(OH)D [median (Q1,Q3) 22.6 (15.8, 26.9) vs. 31.1 (23.1, 38.8) ng/ml, P < 0.001] but higher bioavailable 25-(OH)D [2.9 (1.5, 5.2) vs. 2.0 (1.5, 3.0) ng/ml, P = 0.022] than nonblack participants. After 48 weeks of VitD/calcium supplementation, bioavailable 25-(OH)D increased more in black than nonblack participants, but there were no between-race differences change in bone turnover markers or bone mineral density. The associations between increases in 25-(OH)D levels and change in bone outcomes appeared similar for both total and bioavailable 25-(OH)D.
Conclusion: Baseline and change in bioavailable 25-(OH)D were higher among black adults initiating antiretroviral therapy with VitD/calcium; however, associations between 25-(OH)D and bone outcomes appeared similar for total and bioavailable 25-(OH)D. The assessment of total 25-(OH)D may be sufficient for evaluation of VitD status in black HIV-infected individuals.
Trial registration number: NCT01403051.
Conflict of interest statement
Figures

References
-
- Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–959. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

