Opposite Roles of RNase and Kinase Activities of Inositol-Requiring Enzyme 1 (IRE1) on HSV-1 Replication
- PMID: 28832521
- PMCID: PMC5618002
- DOI: 10.3390/v9090235
Opposite Roles of RNase and Kinase Activities of Inositol-Requiring Enzyme 1 (IRE1) on HSV-1 Replication
Abstract
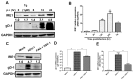
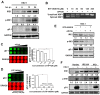
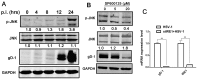
In response to the endoplasmic reticulum (ER) stress induced by herpes simplex virus type 1 (HSV-1) infection, host cells activate the unfolded protein response (UPR) to reduce the protein-folding burden in the ER. The regulation of UPR upon HSV-1 infection is complex, and the downstream effectors can be detrimental to viral replication. Therefore, HSV-1 copes with the UPR to create a beneficial environment for its replication. UPR has three branches, including protein kinase RNA (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activated transcription factor 6 (ATF6). IRE1α is the most conserved branch of UPR which has both RNase and kinase activities. Previous studies have shown that IRE1α RNase activity was inactivated during HSV-1 infection. However, the effect of the two activities of IRE1α on HSV-1 replication remains unknown. Results in this study showed that IRE1α expression was up-regulated during HSV-1 infection. We found that in HEC-1-A cells, increasing RNase activity, or inhibiting kinase activity of IRE1α led to viral suppression, indicating that the kinase activity of IRE1α was beneficial, while the RNase activity was detrimental to viral replication. Further evidence showed that the kinase activity of IRE1α leads to the activation of the JNK (c-Jun N-terminal kinases) pathway, which enhances viral replication. Taken together, our evidence suggests that IRE1α is involved in HSV-1 replication, and its RNase and kinase activities play differential roles during viral infection.
Keywords: X-box binding protein 1 (XBP1); endoplasmic reticulum (ER); herpes simplex virus 1 (HSV-1); inositol-requiring enzyme 1 (IRE1); unfolded protein response (UPR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Herpes Simplex Virus 1 UL41 Protein Suppresses the IRE1/XBP1 Signal Pathway of the Unfolded Protein Response via Its RNase Activity.J Virol. 2017 Jan 31;91(4):e02056-16. doi: 10.1128/JVI.02056-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27928013 Free PMC article.
-
ER stress and distinct outputs of the IRE1α RNase control proliferation and senescence in response to oncogenic Ras.Proc Natl Acad Sci U S A. 2017 Sep 12;114(37):9900-9905. doi: 10.1073/pnas.1701757114. Epub 2017 Aug 28. Proc Natl Acad Sci U S A. 2017. PMID: 28847931 Free PMC article.
-
Inositol-Requiring Enzyme 1α Promotes Zika Virus Infection through Regulation of Stearoyl Coenzyme A Desaturase 1-Mediated Lipid Metabolism.J Virol. 2020 Nov 9;94(23):e01229-20. doi: 10.1128/JVI.01229-20. Print 2020 Nov 9. J Virol. 2020. PMID: 32967957 Free PMC article.
-
The multiple roles of the unfolded protein response regulator IRE1α in cancer.Mol Carcinog. 2019 Sep;58(9):1623-1630. doi: 10.1002/mc.23031. Epub 2019 Apr 30. Mol Carcinog. 2019. PMID: 31041814 Free PMC article. Review.
-
Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease.J Biol Chem. 2019 Dec 6;294(49):18726-18741. doi: 10.1074/jbc.REV119.007036. Epub 2019 Oct 30. J Biol Chem. 2019. PMID: 31666338 Free PMC article. Review.
Cited by
-
Manipulation of unfolded protein response by zoonotic vaccinia virus strains Guarani P1 and Passatempo.Exp Biol Med (Maywood). 2023 Oct;248(19):1684-1693. doi: 10.1177/15353702231211857. Epub 2023 Nov 29. Exp Biol Med (Maywood). 2023. PMID: 38031237 Free PMC article.
-
Induction of the Unfolded Protein Response during Bovine Alphaherpesvirus 1 Infection.Viruses. 2020 Sep 2;12(9):974. doi: 10.3390/v12090974. Viruses. 2020. PMID: 32887282 Free PMC article.
-
Marburg virus regulates the IRE1/XBP1-dependent unfolded protein response to ensure efficient viral replication.Emerg Microbes Infect. 2019;8(1):1300-1313. doi: 10.1080/22221751.2019.1659552. Emerg Microbes Infect. 2019. PMID: 31495285 Free PMC article.
-
Host shutoff activity of VHS and SOX-like proteins: role in viral survival and immune evasion.Virol J. 2020 May 19;17(1):68. doi: 10.1186/s12985-020-01336-8. Virol J. 2020. PMID: 32430029 Free PMC article. Review.
-
Anti-viral activity of Staphylococcus aureus lysates against herpes simplex virus type-I infection: an in vitro and in vivo study.Int J Ophthalmol. 2021 Oct 18;14(10):1463-1472. doi: 10.18240/ijo.2021.10.01. eCollection 2021. Int J Ophthalmol. 2021. PMID: 34667721 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

