Contribution of Intrinsic Lactate to Maintenance of Seizure Activity in Neocortical Slices from Patients with Temporal Lobe Epilepsy and in Rat Entorhinal Cortex
- PMID: 28832554
- PMCID: PMC5618484
- DOI: 10.3390/ijms18091835
Contribution of Intrinsic Lactate to Maintenance of Seizure Activity in Neocortical Slices from Patients with Temporal Lobe Epilepsy and in Rat Entorhinal Cortex
Abstract
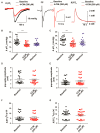
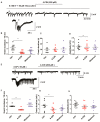
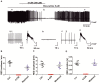
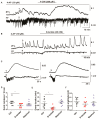
Neuronal lactate uptake supports energy metabolism associated with synaptic signaling and recovery of extracellular ion gradients following neuronal activation. Altered expression of the monocarboxylate transporters (MCT) in temporal lobe epilepsy (TLE) hampers lactate removal into the bloodstream. The resulting increase in parenchymal lactate levels might exert both, anti- and pro-ictogen effects, by causing acidosis and by supplementing energy metabolism, respectively. Hence, we assessed the contribution of lactate to the maintenance of transmembrane potassium gradients, synaptic signaling and pathological network activity in chronic epileptic human tissue. Stimulus induced and spontaneous field potentials and extracellular potassium concentration changes (∆[K⁺]O) were recorded in parallel with tissue pO₂ and pH in slices from TLE patients while blocking MCTs by α-cyano-4-hydroxycinnamic acid (4-CIN) or d-lactate. Intrinsic lactate contributed to the oxidative energy metabolism in chronic epileptic tissue as revealed by the changes in pO₂ following blockade of lactate uptake. However, unlike the results in rat hippocampus, ∆[K⁺]O recovery kinetics and field potential amplitude did not depend on the presence of lactate. Remarkably, inhibition of lactate uptake exerted pH-independent anti-seizure effects both in healthy rat and chronic epileptic tissue and this effect was partly mediated via adenosine 1 receptor activation following decreased oxidative metabolism.
Keywords: adenosine; interictal activity; lactate; mesial temporal lobe epilepsy; monocarboxylate transporter inhibitors; seizure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bröer S., Rahman B., Pellegri G., Pellerin L., Martin J.L., Verleysdonk S., Hamprecht B., Magistretti P.J. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem. 1997;272:30096–30102. doi: 10.1074/jbc.272.48.30096. - DOI - PubMed
-
- Sotelo-Hitschfeld T., Niemeyer M.I., Machler P., Ruminot I., Lerchundi R., Wyss M.T., Stobart J., Fernández-Moncada I., Valdebenito R., Garrido-Gerter P., et al. Channel-Mediated Lactate Release by K+-Stimulated Astrocytes. J. Neurosci. 2015;35:4168–41478. doi: 10.1523/JNEUROSCI.5036-14.2015. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

