Uptake of genetic testing by the children of Lynch syndrome variant carriers across three generations
- PMID: 28832568
- PMCID: PMC5643966
- DOI: 10.1038/ejhg.2017.132
Uptake of genetic testing by the children of Lynch syndrome variant carriers across three generations
Abstract
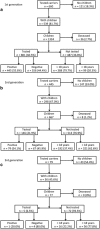
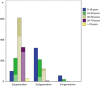
Many Lynch syndrome (LS) carriers remain unidentified, thus missing early cancer detection and prevention opportunities. Tested probands should inform their relatives about cancer risk and options for genetic counselling and predictive gene testing, but many fail to undergo testing. To assess predictive testing uptake and demographic factors influencing this decision in LS families, a cross-sectional registry-based cohort study utilizing the Finnish Lynch syndrome registry was undertaken. Tested LS variant probands (1184) had 2068 children divided among three generations: 660 parents and 1324 children (first), 445 and 667 (second), and 79 and 77 (third). Of children aged >18 years, 801 (67.4%), 146 (43.2%), and 5 (23.8%), respectively, were genetically tested. Together, 539 first-generation LS variant carriers had 2068 children and grandchildren (3.84 per carrier). Of the 1548 (2.87 per carrier) eligible children, 952 (61.5%) were tested (1.77 per carrier). In multivariate models, age (OR 1.08 per year; 95% CI 1.06-1.10), family gene (OR 2.83; 1.75-4.57 for MLH1 and 2.59; 1.47-4.56 for MSH2 compared with MSH6), one or more tested siblings (OR 6.60; 4.82-9.03), no siblings (OR 4.63; 2.64-8.10), and parent under endoscopic surveillance (OR 5.22; 2.41-11.31) were independent predictors of having genetic testing. Examples of parental adherence to regular surveillance and genetically tested siblings strongly influenced children at 50% risk of LS to undergo predictive gene testing. High numbers of untested, adult at-risk individuals exist even among well-established cohorts of known LS families with good adherence to endoscopic surveillance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Giardiello FM, Allen JI, Axilbund JE et al: Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol 2014; 109: 1159–1179. - PubMed
-
- Stoffel EM, Mangu PB, Gruber SB et al: Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the Familial Risk-Colorectal Cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol 2014; 11: e437–e441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous