Cost-effectiveness of Evolocumab Therapy for Reducing Cardiovascular Events in Patients With Atherosclerotic Cardiovascular Disease
- PMID: 28832867
- PMCID: PMC5710446
- DOI: 10.1001/jamacardio.2017.2762
Cost-effectiveness of Evolocumab Therapy for Reducing Cardiovascular Events in Patients With Atherosclerotic Cardiovascular Disease
Erratum in
-
Error in Figure.JAMA Cardiol. 2017 Oct 1;2(10):1170. doi: 10.1001/jamacardio.2017.3817. JAMA Cardiol. 2017. PMID: 29049503 Free PMC article. No abstract available.
Abstract
Importance: The proprotein convertase subtilisin/kexin type 9 inhibitor evolocumab has been demonstrated to reduce the composite of myocardial infarction, stroke, or cardiovascular death in patients with established atherosclerotic cardiovascular disease. To our knowledge, long-term cost-effectiveness of this therapy has not been evaluated using clinical trial efficacy data.
Objective: To evaluate the cost-effectiveness of evolocumab in patients with atherosclerotic cardiovascular disease when added to standard background therapy.
Design, setting, and participants: A Markov cohort state-transition model was used, integrating US population-specific demographics, risk factors, background therapy, and event rates along with trial-based event risk reduction. Costs, including price of drug, utilities, and transitional probabilities, were included from published sources.
Exposures: Addition of evolocumab to standard background therapy including statins.
Main outcomes and measures: Cardiovascular events including myocardial infarction, ischemic stroke and cardiovascular death, quality-adjusted life-year (QALY), incremental cost-effectiveness ratio (ICER), and net value-based price.
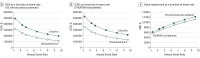
Results: In the base case, using US clinical practice patients with atherosclerotic cardiovascular disease with low-density lipoprotein cholesterol levels of at least 70 mg/dL (to convert to millimoles per liter, multiply by 0.0259) and an annual events rate of 6.4 per 100 patient-years, evolocumab was associated with increased cost and improved QALY: incremental cost, $105 398; incremental QALY, 0.39, with an ICER of $268 637 per QALY gained ($165 689 with discounted price of $10 311 based on mean rebate of 29% for branded pharmaceuticals). Sensitivity and scenario analyses demonstrated ICERs ranging from $100 193 to $488 642 per QALY, with ICER of $413 579 per QALY for trial patient characteristics and event rate of 4.2 per 100 patient-years ($270 192 with discounted price of $10 311) and $483 800 if no cardiovascular mortality reduction emerges. Evolocumab treatment exceeded $150 000 per QALY in most scenarios but would meet this threshold at an annual net price of $9669 ($6780 for the trial participants) or with the discounted net price of $10 311 in patients with low-density lipoprotein cholesterol levels of at least 80 mg/dL.
Conclusions and relevance: At its current list price of $14 523, the addition of evolocumab to standard background therapy in patients with atherosclerotic cardiovascular disease exceeds generally accepted cost-effectiveness thresholds. To achieve an ICER of $150 000 per QALY, the annual net price would need to be substantially lower ($9669 for US clinical practice and $6780 for trial participants), or a higher-risk population would need to be treated.
Conflict of interest statement
Figures
Comment in
-
Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor Therapy-Breakthrough in Low-Density Lipoprotein Cholesterol Lowering, Breakdown in Value.JAMA Cardiol. 2017 Oct 1;2(10):1066-1068. doi: 10.1001/jamacardio.2017.2911. JAMA Cardiol. 2017. PMID: 28832860 No abstract available.
-
Cost-effectiveness of PCSK9 Inhibitors: Proof in the Modeling.JAMA Cardiol. 2017 Dec 1;2(12):1298-1299. doi: 10.1001/jamacardio.2017.3656. JAMA Cardiol. 2017. PMID: 29049827 No abstract available.
References
-
- Davis KL, Meyers J, Zhao Z, McCollam PL, Murakami M. High-risk atherosclerotic cardiovascular disease in a real-world employed Japanese population: prevalence, cardiovascular event rates, and costs. J Atheroscler Thromb. 2015;22(12):1287-1304. - PubMed
-
- Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015;36(19):1163-1170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

