Effect of Intravenously Administered Crystalloid Solutions on Acid-Base Balance in Domestic Animals
- PMID: 28833697
- PMCID: PMC5598900
- DOI: 10.1111/jvim.14803
Effect of Intravenously Administered Crystalloid Solutions on Acid-Base Balance in Domestic Animals
Abstract
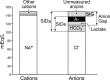
Intravenous fluid therapy can alter plasma acid-base balance. The Stewart approach to acid-base balance is uniquely suited to identify and quantify the effects of the cationic and anionic constituents of crystalloid solutions on plasma pH. The plasma strong ion difference (SID) and weak acid concentrations are similar to those of the administered fluid, more so at higher administration rates and with larger volumes. A crystalloid's in vivo effects on plasma pH are described by 3 general rules: SID > [HCO3-] increases plasma pH (alkalosis); SID < [HCO3-] decreases plasma pH (alkalosis); and SID = [HCO3-] yields no change in plasma pH. The in vitro pH of commercially prepared crystalloid solutions has little to no effect on plasma pH because of their low titratable acidity. Appreciation of IV fluid composition and an understanding of basic physicochemical principles provide therapeutically valuable insights about how and why fluid therapy can produce and correct alterations of plasma acid-base equilibrium. The ideal balanced crystalloid should (1) contain species-specific concentrations of key electrolytes (Na+ , Cl- , K+ , Ca++ , Mg++ ), particularly Na+ and Cl- ; (2) maintain or normalize acid-base balance (provide an appropriate SID); and (3) be isosmotic and isotonic (not induce inappropriate fluid shifts) with normal plasma.
Keywords: Acid-base balance; Base replacement; Fluid therapy; Metabolic acidosis; Physiology.
Copyright © 2017 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals, Inc. on behalf of the American College of Veterinary Internal Medicine.
Figures


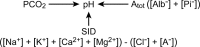
References
-
- Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med 2013;369:1243–1251. - PubMed
-
- Raghunathan K, Shaw AD, Bagshaw SM. Fluids are drugs: Type, dose and toxicity. Curr Opin Crit Care 2013;19:280–298. - PubMed
-
- James MFM. Context‐sensitive fluid administration: What, when and how much. South Afr J Anaesth Analg 2015;21:38–39.
-
- Davis H, Jensen T, Johnson A, et al. American Association of Feline Practictioners; American Animal Hospital Association. 2013 AAHA/AAFP Fluid therapy guidelines for dogs and cats. J Am Animal Hosp Assoc 2013;49:149–159. - PubMed
-
- Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr 2008;27:170–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

