Amyloidogenesis of Tau protein
- PMID: 28833749
- PMCID: PMC5654847
- DOI: 10.1002/pro.3275
Amyloidogenesis of Tau protein
Abstract
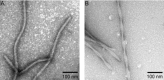
The role of microtubule-associated protein Tau in neurodegeneration has been extensively investigated since the discovery of Tau amyloid aggregates in the brains of patients with Alzheimer's disease (AD). The process of formation of amyloid fibrils is known as amyloidogenesis and attracts much attention as a potential target in the prevention and treatment of neurodegenerative conditions linked to protein aggregation. Cerebral deposition of amyloid aggregates of Tau is observed not only in AD but also in numerous other tauopathies and prion diseases. Amyloidogenesis of intrinsically unstructured monomers of Tau can be triggered by mutations in the Tau gene, post-translational modifications, or interactions with polyanionic molecules and aggregation-prone proteins/peptides. The self-assembly of amyloid fibrils of Tau shares a number of characteristic features with amyloidogenesis of other proteins involved in neurodegenerative diseases. For example, in vitro experiments have demonstrated that the nucleation phase, which is the rate-limiting stage of Tau amyloidogenesis, is shortened in the presence of fragmented preformed Tau fibrils acting as aggregation templates ("seeds"). Accordingly, Tau aggregates released by tauopathy-affected neurons can spread the neurodegenerative process in the brain through a prion-like mechanism, originally described for the pathogenic form of prion protein. Moreover, Tau has been shown to form amyloid strains-structurally diverse self-propagating aggregates of potentially various pathological effects, resembling in this respect prion strains. Here, we review the current literature on Tau aggregation and discuss mechanisms of propagation of Tau amyloid in the light of the prion-like paradigm.
Keywords: Alzheimer's disease; Tau protein; amyloidogenesis; protein aggregation; tauopathies.
© 2017 The Protein Society.
Figures



References
-
- Rüb U, Stratmann K, Heinsen H, Seidel K, Bouzrou M, Korf HW (2017) Alzheimer's disease: characterization of the brain sites of the initial Tau cytoskeletal pathology will improve the success of novel immunological anti‐Tau treatment approaches. J Alzheimers Dis 57:683–696. - PubMed
-
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297:353–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

