Multinodular and vacuolating neuronal tumors in epilepsy: dysplasia or neoplasia?
- PMID: 28833756
- PMCID: PMC5887881
- DOI: 10.1111/bpa.12555
Multinodular and vacuolating neuronal tumors in epilepsy: dysplasia or neoplasia?
Abstract
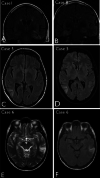
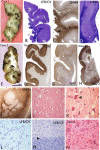
Multinodular and vacuolating neuronal tumor (MVNT) is a new pattern of neuronal tumour included in the recently revised WHO 2016 classification of tumors of the CNS. There are 15 reports in the literature to date. They are typically associated with late onset epilepsy and a neoplastic vs. malformative biology has been questioned. We present a series of ten cases and compare their pathological and genetic features to better characterized epilepsy-associated malformations including focal cortical dysplasia type II (FCDII) and low-grade epilepsy-associated tumors (LEAT). Clinical and neuroradiology data were reviewed and a broad immunohistochemistry panel was applied to explore neuronal and glial differentiation, interneuronal populations, mTOR pathway activation and neurodegenerative changes. Next generation sequencing was performed for targeted multi-gene analysis to identify mutations common to epilepsy lesions including FCDII and LEAT. All of the surgical cases in this series presented with seizures, and were located in the temporal lobe. There was a lack of any progressive changes on serial pre-operative MRI and a mean age at surgery of 45 years. The vacuolated cells of the lesion expressed mature neuronal markers (neurofilament/SMI32, MAP2, synaptophysin). Prominent labelling of the lesional cells for developmentally regulated proteins (OTX1, TBR1, SOX2, MAP1b, CD34, GFAPδ) and oligodendroglial lineage markers (OLIG2, SMI94) was observed. No mutations were detected in the mTOR pathway genes, BRAF, FGFR1 or MYB. Clinical, pathological and genetic data could indicate that MVNT aligns more with a malformative lesion than a true neoplasm with origin from a progenitor neuro-glial cell type showing aberrant maturation.
Keywords: Multinodular; epilepsy; neuronal; tumour; vacuolating.
© 2017 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Figures


References
-
- Aronica E, Boer K, Redeker S, Spliet WG, van Rijen PC, Troost D, Gorter JA (2007) Differential expression patterns of chloride transporters, Na+‐K+‐2Cl – cotransporter and K+‐Cl – cotransporter, in epilepsy‐associated malformations of cortical development. Neuroscience 145:185–196. - PubMed
-
- Avanzini G, Spreafico R, Cipelletti B, Sancini G, Frassoni C, Franceschetti S et al (2000) Synaptic properties of neocortical neurons in epileptic mice lacking the Otx1 gene. Epilepsia 41:S200–S205. - PubMed
-
- Badat N, Savatovsky J, Charbonneau F, Collin A, Lecler A (2017) Multinodular vacuolating and neuronal tumor of the cerebrum. Neurology 89:304–305. - PubMed
-
- Baulac S, Ishida S, Marsan E, Miquel C, Biraben A, Nguyen DK et al (2015) Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann Neurol 77:675–683. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

