Periostin promotes migration and osteogenic differentiation of human periodontal ligament mesenchymal stem cells via the Jun amino-terminal kinases (JNK) pathway under inflammatory conditions
- PMID: 28833827
- PMCID: PMC6529146
- DOI: 10.1111/cpr.12369
Periostin promotes migration and osteogenic differentiation of human periodontal ligament mesenchymal stem cells via the Jun amino-terminal kinases (JNK) pathway under inflammatory conditions
Abstract
Objectives: Mesenchymal stem cell (MSC)-mediated periodontal tissue regeneration is considered to be a promising method for periodontitis treatment. The molecular mechanism of functional regulation by MSCs remains unclear, thus limiting their application. Our previous study discovered that Periostin (POSTN) promoted the migration and osteogenic differentiation of periodontal ligament mesenchymal stem cells (PDLSCs), but it is still unclear whether POSTN is able to restore the regenerative potential of PDLSCs under inflammatory conditions. In this study, we investigated the effect of POSTN on PDLSCs under inflammatory conditions and its mechanism.
Materials and methods: PDLSCs were isolated from periodontal ligament tissue. TNF-α was used at 10 ng/mL to mimic inflammatory conditions. Lentivirus POSTN shRNA was used to knock down POSTN. Recombinant human POSTN (rhPOSTN) was used to stimulate PDLSCs. A scratch assay was used to analyse cell migration. Alkaline phosphatase (ALP) activity, Alizarin Red staining and expression of osteogenesis-related genes were used to investigate the osteogenic differentiation potential. Western blot analysis was used to detect the mitogen-activated protein kinases (MAPK) and AKT signalling pathways.
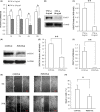
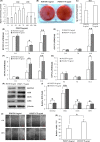
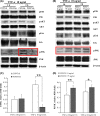
Results: After a 10 ng/mL TNF-α treatment, knockdown of POSTN impeded scratch closure, inhibited ALP activity and mineralization in vitro, and decreased expression of RUNX2, OSX, OPN and OCN in PDLSCs, while 75 ng/mL rhPOSTN significantly accelerated scratch closure, enhanced ALP activity and mineralization in vitro, and increased expression of RUNX2, OSX, OPN and OCN. In addition, knockdown of POSTN inhibited expression of phosphorylated c-Jun N-terminal kinase (p-JNK), while 75 ng/mL rhPOSTN increased expression of p-JNK in PDLSCs with TNF-α treatment. Furthermore, inhibition of JNK by its inhibitor SP600125 dramatically blocked POSTN-enhanced scratch closure, ALP activity and mineralization in PDLSCs.
Conclusions: Our results revealed that POSTN might promote the migration and osteogenic differentiation potential of PDLSCs via the JNK pathway, providing insight into the mechanism underlying MSC biology under inflammatory conditions and identifying a potential target for improving periodontal tissue regeneration.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this manuscript.
Figures


References
-
- Liu Y, Hu J, Wang S. Mesenchymal stem cell‐mediated treatment of oral diseases. Histol Histopathol. 2014;29:1007‐1015. - PubMed
-
- Yam GH, Peh GS, Singhal S, Goh BT, Mehta JS. Dental stem cells: a future asset of ocular cell therapy. Expert Rev Mol Med. 2015;17:e20. - PubMed
-
- Wu Z, Dai W, Wang P, et al. Periostin promotes migration, proliferation, and differentiation of human periodontal ligament mesenchymal stem cells. Connect Tissue Res. 2017;1. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

