Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review
- PMID: 28834075
- PMCID: PMC5813232
- DOI: 10.1111/dom.13088
Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review
Abstract
Aims: Therapeutic inertia, defined as the failure to initiate or intensify therapy in a timely manner according to evidence-based clinical guidelines, is a key reason for uncontrolled hyperglycaemia in patients with type 2 diabetes. The aims of this systematic review were to identify how therapeutic inertia in the management of hyperglycaemia was measured and to assess its extent over the past decade.
Materials and methods: Systematic searches for articles published from January 1, 2004 to August 1, 2016 were conducted in MEDLINE and Embase. Two researchers independently screened all of the titles and abstracts, and the full texts of publications deemed relevant. Data were extracted by a single researcher using a standardized data extraction form.
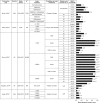
Results: The final selection for the review included 53 articles. Measurements used to assess therapeutic inertia varied across studies, making comparisons difficult. Data from low- to middle-income countries were scarce. In most studies, the median time to treatment intensification after a glycated haemoglobin (HbA1c) measurement above target was more than 1 year (range 0.3 to >7.2 years). Therapeutic inertia increased as the number of antidiabetic drugs rose and decreased with increasing HbA1c levels. Data were mainly available from Western countries. Diversity of inertia measures precluded meta-analysis.
Conclusions: Therapeutic inertia in the management of hyperglycaemia in patients with type 2 diabetes is a major concern. This is well documented in Western countries, but corresponding data are urgently needed in low- and middle-income countries, in view of their high prevalence of type 2 diabetes.
Keywords: antidiabetic drug; glycaemic control; systematic review; type 2 diabetes.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
K. K. has received honoraria and research grants from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, Sanofi‐Aventis, Takeda, Bristol‐Myers Squibb and Unilever. K. K. also acknowledges the support of the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care—East Midlands (NIHR CLAHRC—EM) and the National Institute of Health Research (NIHR) Leicester–Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit. M. B. G. has received honoraria from AstraZeneca and Merck‐Serono. S. Po. has received honoraria from AstraZeneca. M. V. S. has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharpe & Dohme, Novartis, Novo Nordisk, Sanofi and Servier, and has received research support from Sanofi. S. Pi. is an employee of Oxford PharmaGenesis, which received funding from AstraZeneca. P. F., N. H. and J. M. are employees of AstraZeneca.
Figures




References
-
- Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta‐analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. - PubMed
-
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐Year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. - PubMed
-
- UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. - PubMed
-
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

