Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cells: Preclinical Efficacy and Safety in Cervical Spinal Cord Injury
- PMID: 28834391
- PMCID: PMC6430160
- DOI: 10.1002/sctm.17-0065
Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cells: Preclinical Efficacy and Safety in Cervical Spinal Cord Injury
Abstract
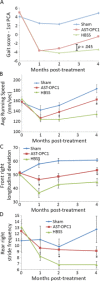
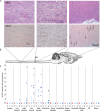
Cervical spinal cord injury (SCI) remains an important research focus for regenerative medicine given the potential for severe functional deficits and the current lack of treatment options to augment neurological recovery. We recently reported the preclinical safety data of a human embryonic cell-derived oligodendrocyte progenitor cell (OPC) therapy that supported initiation of a phase I clinical trial for patients with sensorimotor complete thoracic SCI. To support the clinical use of this OPC therapy for cervical injuries, we conducted preclinical efficacy and safety testing of the OPCs in a nude rat model of cervical SCI. Using the automated TreadScan system to track motor behavioral recovery, we found that OPCs significantly improved locomotor performance when administered directly into the cervical spinal cord 1 week after injury, and that this functional improvement was associated with reduced parenchymal cavitation and increased sparing of myelinated axons within the injury site. Based on large scale biodistribution and toxicology studies, we show that OPC migration is limited to the spinal cord and brainstem and did not cause any adverse clinical observations, toxicities, allodynia, or tumors. In combination with previously published efficacy and safety data, the results presented here supported initiation of a phase I/IIa clinical trial in the U.S. for patients with sensorimotor complete cervical SCI. Stem Cells Translational Medicine 2017;6:1917-1929.
Keywords: Cervical spinal cord injury; Clinical trial; Human embryonic stem cell; Oligodendrocyte progenitor cell; Preclinical; Spinal cord injury.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
C.A.P., J.D., E.D.W., and J.S.L. were employees of Geron Corporation when the studies were conducted. N.C.M., E.D.W., and J.S.L. are employed by Asterias Biotherapeutics, Inc. In the U.S. and internationally, patents have been filed and issued for AST‐OPC1. The authors have no other relevant affiliations or financial involvement with any organizations or entities with a potential conflict of interest with any of the subject matter in this manuscript apart from those disclosed.
Figures


References
-
- Kakulas BA. The applied neuropathology of human spinal cord injury. Spinal Cord 1999;37:79–88. - PubMed
-
- Anderson DK, Hall ED. Pathophysiology of spinal cord trauma. Ann Emerg Med 1993;22:987–992. - PubMed
-
- Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: Defining the problems. J Neurotrauma 2004;21:429–440. - PubMed
-
- Anderson KD, Fridén J, Lieber RL. Acceptable benefits and risks associated with surgically improving arm function in individuals living with cervical spinal cord injury. Spinal Cord 2009;47:334–338. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

